The CDRC has a comprehensive system for ensuring medication is appropriately monitored and safely administered, based on information in the BNF, national and local guidelines.
Medication is divided into three basic categories with respect to monitoring:
- Medication that is almost always prescribed in secondary care (e.g. biologics)
- Medications are taken into consideration when looking at factors such as drug interactions or immunosuppression
- An assumption is made that appropriate monitoring is the responsibility of the prescriber – they are not included in the monitoring system
- Medication that might be prescribed in primary care or elsewhere (e.g. DMARD at initiation, antipsychotics)
- Medications are taken into consideration when looking at factors such as drug interactions or immunosuppression
- These medications are included in the monitoring system if they are on repeat prescription AND the medication has been issued from primary care recently (usually in the last three months)
- Medication that is almost always prescribed in primary care (e.g. ACE inhibitors, anticoagulants)
- Medications are taken into consideration when looking at factors such as drug interactions or immunosuppression
- These medications are included in the monitoring system if they are on repeat prescription irrespective of recent issues. (There are separate systems in place to detect important medication on repeat that is not being issued).
In general, only medication on repeat templates are included in the monitoring system with a few exceptions such as people receiving repeated NSAIDs. We strongly recommend that all medication which is being taken regularly by the patient is included in a repeat template. Medication that is not being issued in primary care can be set so that it is extremely unlikely that it can be issued (see Medication Prescribed Elsewhere).
Available Resources:
Reports
A comprehensive set of reports is available to identify patients who are due or overdue drug monitoring:

Reports are also available for individual drugs:

The following time periods are generally used to define monitoring status:
| Usual Monitoring Frequency | Due | Overdue | Significantly overdue | Very significantly overdue |
| Monthly | 21d | 28d | 35d | N/A |
| 2 monthly | 49d | 56d | 63d | N/A |
| 3 monthly | 77d | 84d | 91d | N/A |
| 6 monthly | 5m | 6m | 7m | 9m |
| Annually or longer | 11m Or 1m before | 12m or equivalent | 13m Or 1m after | 18m Or 6m after |
Patient Status Alerts
Patients who are on a drug that needs monitoring will have the follow icon if the monitoring is up to date:

and this icon if the monitoring is due:

Templates
How to Access:
In the lower left hand corner use the search bar, type in ‘Drugs Requiring Monitoring – CDRC’ and select the following template:
Alternatively, press F12 and search for ‘Drugs Requiring Monitoring – CDRC’, this will open the aforementioned template.
The Drugs Requiring Monitoring template shows which medication(s) the patient is on and which tests are due/overdue.
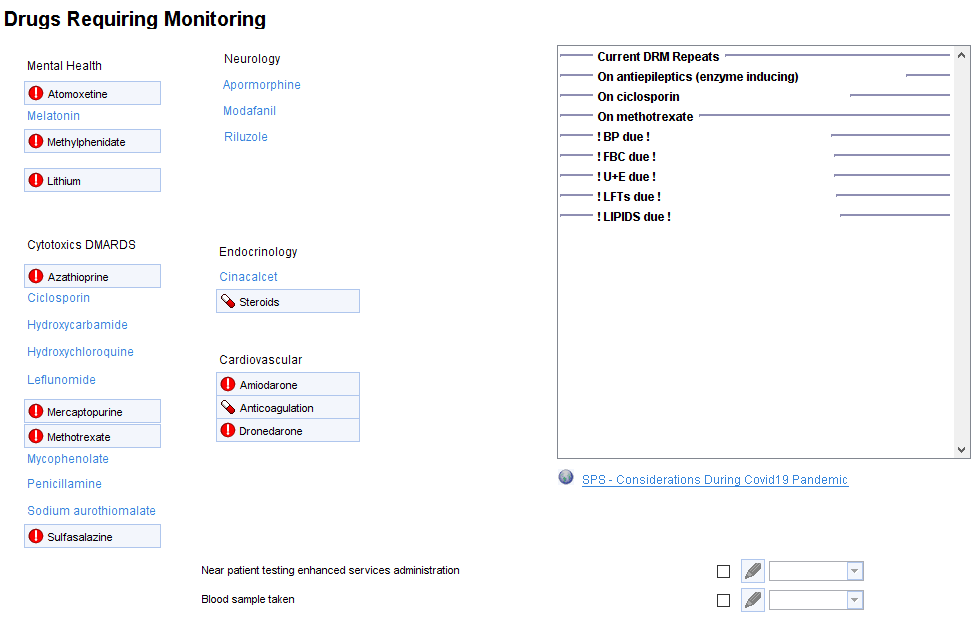
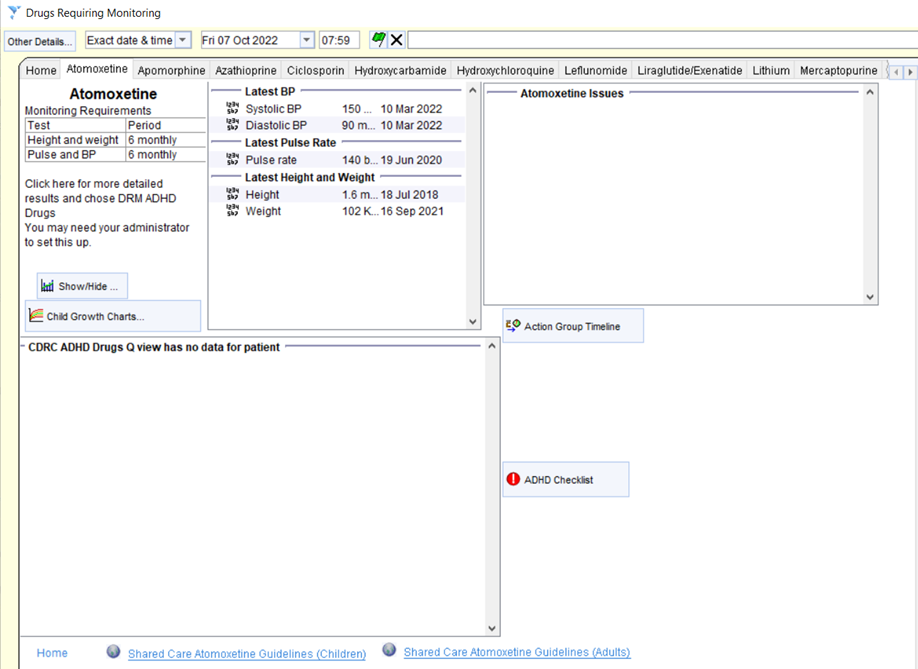
There are links to more detailed information about each medication on separate pages, for example:
The monitoring issues are also shown on the LTC Management Template alongside any other tests that are needed for the long term condition review …….
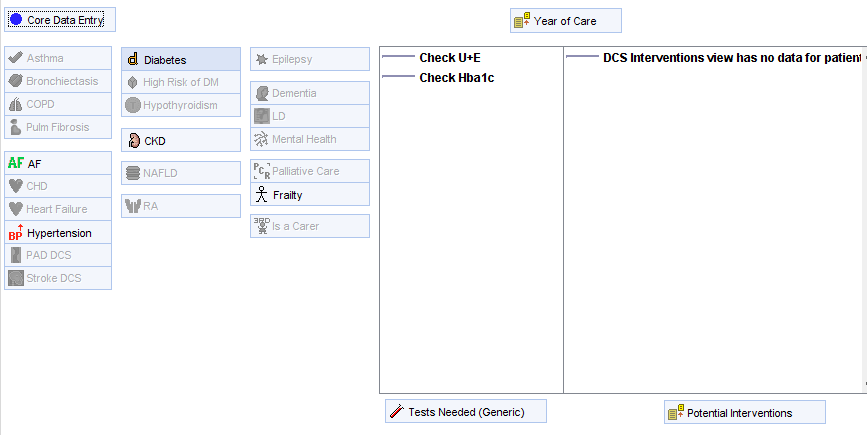
……. And the Medication Review Tool template.
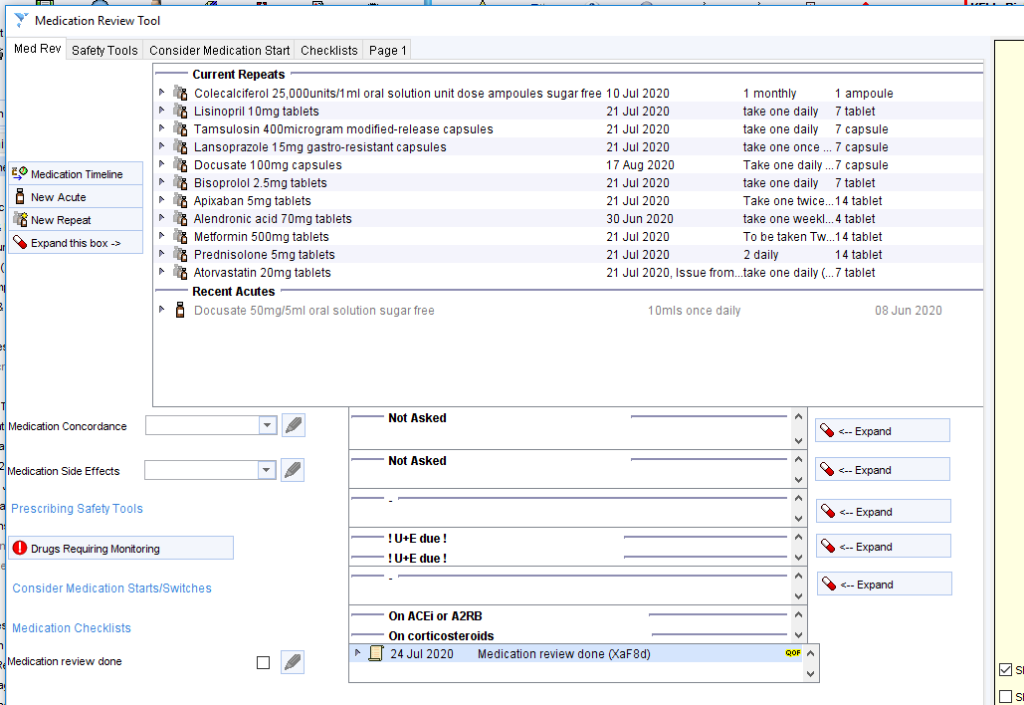
For some medications the monitoring frequency can be customised from the medication specific link e.g. default methotrexate monitoring is monthly but this can be amended to 2 or 3 monthly once stable in line with local guidelines.
Using the System to Call Patients
The system is designed to integrate with long term condition management, so patients are not brought unnecessarily for separate drug monitoring and LTC review appointments. It is also designed to allow units to customise the system for their own requirements.
At the simplest level the ! *DRM and any test due # will show the patients who will be due to have a monitoring test in the near future. ! *DRM and any test overdue # shows those patients who have become due today.
For 6 monthly tests there is a separate report which shows only patients who would not be called for a 6 monthly LTC review.
For annual or less frequent tests there is a separate report to show only patients who do not have one of the common LTCs.
Local Customisation
Individual units can combine the available reports for their own requirements. An example is shown below.
- Appointments for monthly tests are assumed, in this unit, to be booked at the previous appointment so only those who are overdue are added to the call list (as a backstop, rather than for initial call)
- Patients needing 2 or 3 monthly monitoring tests are included in the list a week before their test is due to ensure they can be invited or already have an appointment
- Patients needing 6 monthly tests are invited on the day the test becomes due unless they have a long term condition which means they will be invited for a 6 month review anyway
- Patients needing annual tests are invited on the day the test becomes due unless they have a long term condition which means they will be invited for an annual review anyway.
- There is then a ‘backstop’ report which shows patients with or without an LTC whose monitoring is very significantly overdue – ! *DRM and any test very significantly overdue (6 or 12 monthly tests) #
Further customisation can be achieved with some technical experience. For example by creating a local copy of the ! *DRM LTC Conditions (Common Ones) report to include all the conditions which have LTC reviews at that unit and then a local copy of the linked reports.
Creating more Complex Outputs
It is possible, with some extra work, to create more complex outputs showing information such as:
- Future appointments
- Which tests are due
An example is shown below:
- Create a report with the customised combination of due/overdue/significantly overdue reports such as the example above
- Create a report joining report 1. to future appointments and test due reports like this:
- Create a report joining reports 1. and 2. like this:
![Amend Report
Name
Category
Sub category
*ORM *Leadgate
Drugs requiring monitoring
Z] Add report to favourtes
New Sub-category
Join to two reports
Demographics
Registration
Administration
Child Heath
Risk Factors
Clinical
Report Joining
Join to one report
Join to two reports
Join to more than two reports
Join to report one
Select report one
Clear report
Repon information
00
Join to report two
Select report two
Clear report
Repon information
Join type
Percentage calculation
IN Repon I
IN Repon 2
! •ORM Leadgate - to recall
OCS Qualty Drugs requiring montoring
IN Report 3
IN Repon I
! •ORM Leadgate report and join
DCS Quality Drugs requiring montoring
IN Report 3
Repon on patients fo
the selected reports
• use organisation captation to calculate this repots percentage](https://cdrc.nhs.uk/wp-content/uploads/2022/09/image-260.png)
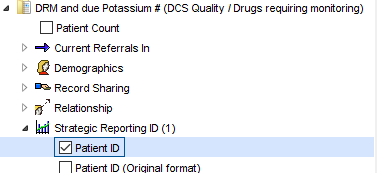
Run report 3 then use the breakdown options to select the features you wish to highlight. E.g. to see which patients need a potassium test:
![DRM and due Potassium # (DCS Quality Drugs requiring montoring)
Patient Count
Current Referrals In
Demographics
Record Sharing
Relationship
Strategic Reporting ID (I)
Z] Patient ID
Patient ID (Oriainal format)](https://cdrc.nhs.uk/wp-content/uploads/2022/09/image-259.png)
To see future appointments:
![Has future appointment (DCS Recall New)
Patient Count
Appointments (2)
Appointment arrival time
Appointment booked date
Appointment branch
Appointment cancellation date
Z] Appointment date
Appointment duration (actual)
[3 Appointment duration (booked)
Appointment flags
[3 Appointment location
Appointment status
[3 Appointment time
Appointment wating time (absolute)
[3 Appointment wating time (calculated)
Booked by
Clinician
e-ReferraI Service appointment
Rota end date](https://cdrc.nhs.uk/wp-content/uploads/2022/09/image-260.png)
