BeatAsthma+
The Beat Asthma+ Project is a feasibility study, aiming to objectively assess a new approach to managing children aged 5-18 years with more difficult asthma in primary care. The Beat Asthma+ Pathway aims to improve outcomes for asthmatic children and ultimately prevent further asthma deaths.
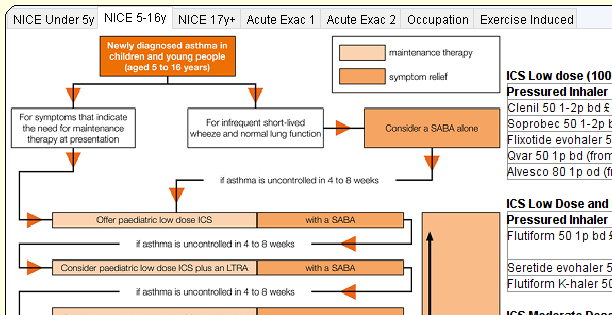
Use of the Beat Asthma+ templates developed by CDRC Precision will enable early identification and management optimisation of children with ‘higher risk’ asthma. The pathway involves the delivery of an intensive education programme to children and their families over a 12 month period and will be implemented across 8 GP surgeries from Newcastle, Gateshead, North Tyneside and South Tyneside CCG’s.
The Primary objective of the study is to demonstrate a reduction in the number of children presenting to hospital with an asthma exacerbation.
Secondary objectives include:
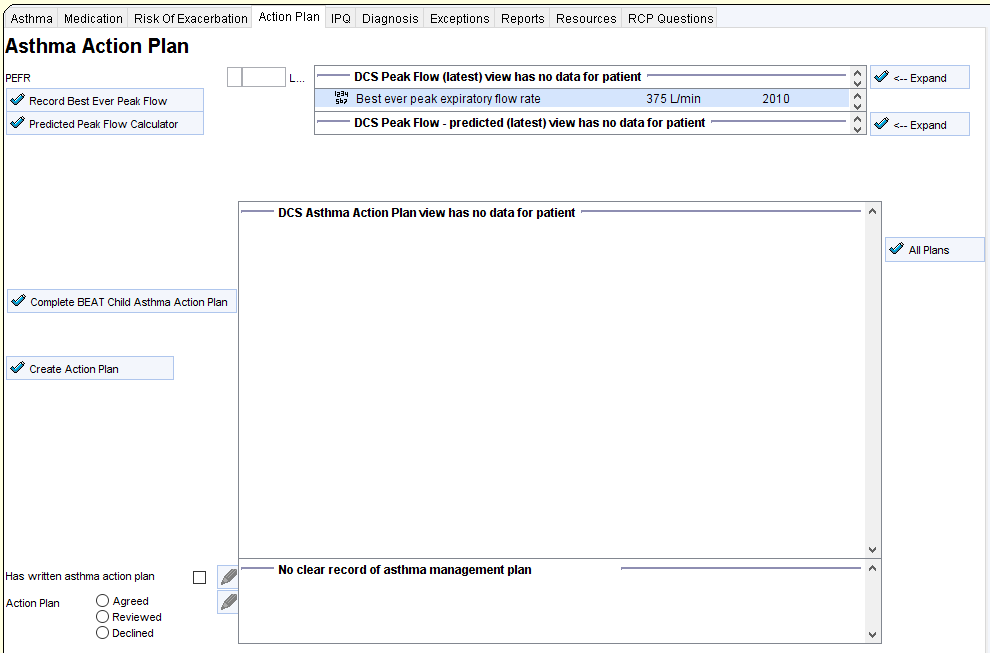
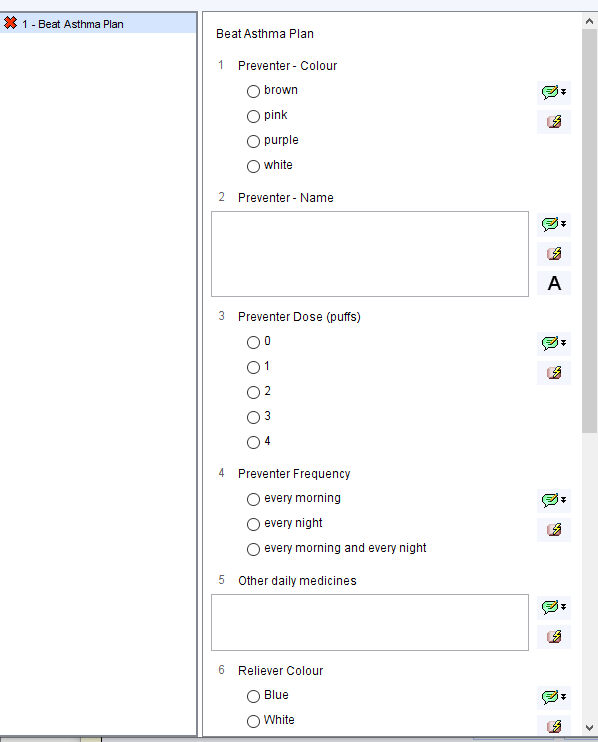
- Improvement in the percentage of patients having a personalised asthma action plan
- Reduction in the average number of reliever inhalers used per patient per year
- Improvement in the average number of preventer inhalers used per patient per year
- Reduction in the average number of courses of steroids used acutely per patient per year
We will also measure:
- Attendance and DNA rates to clinics
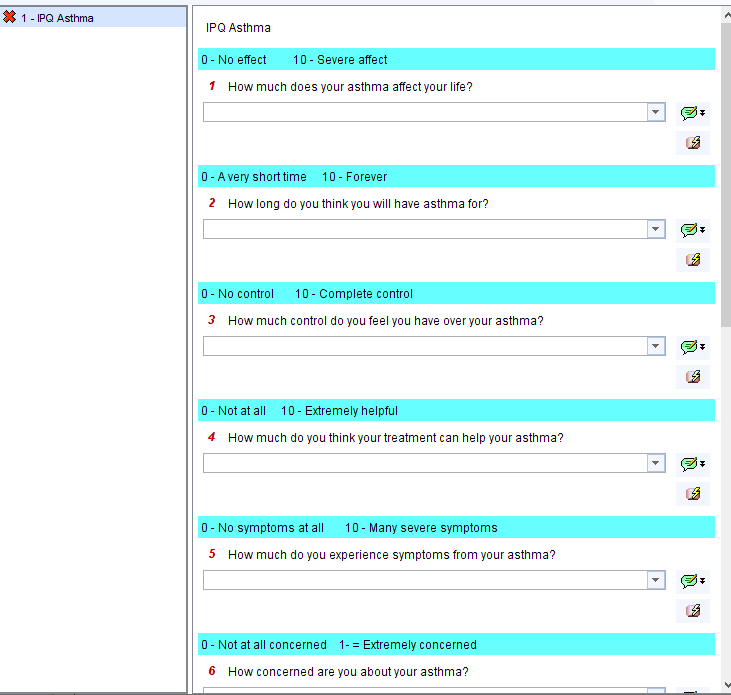
- QOL scores in patients pre and post intervention
Beat Asthma+ Report, Access and Guide
Access:
If you are not already member of the DCS organisational group on SystmOne, please refer to Access Guide here.
Once you have access, you will be able to use the resources highlighted on below.
Guide:
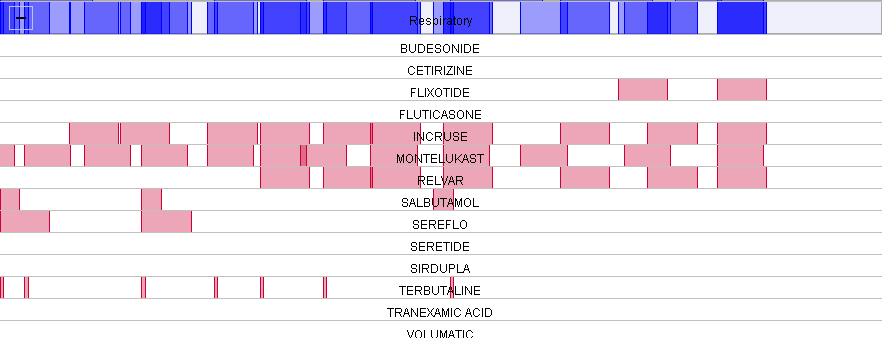
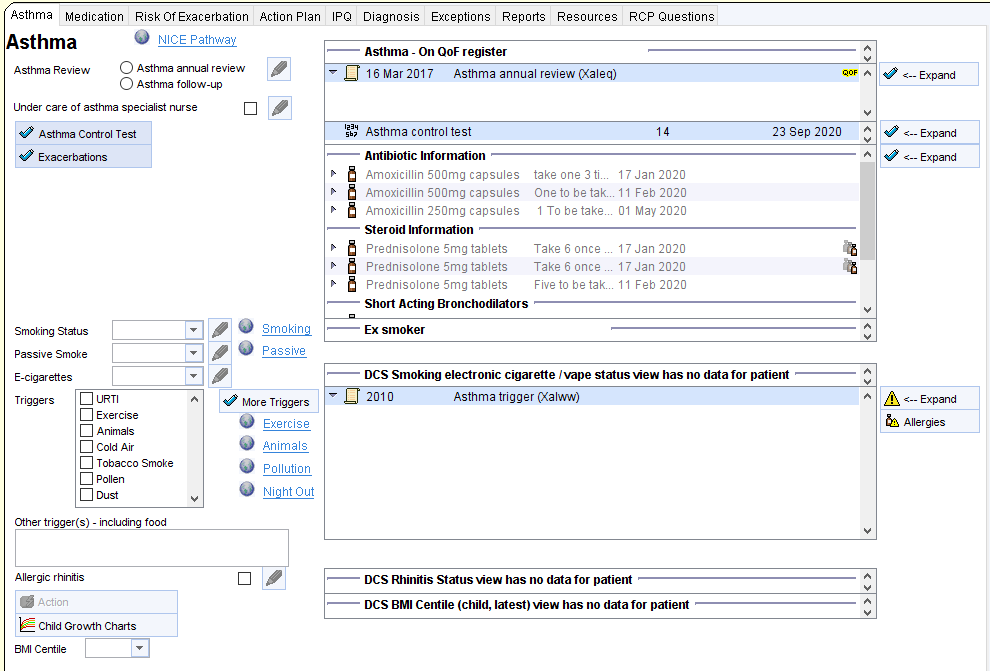
The Beat Asthma+ Report is found in the folder CDRC Quality > Respiratory
It is called ” ? BEATAsthma 1 – Trigger Criteria ” as highlighted below.
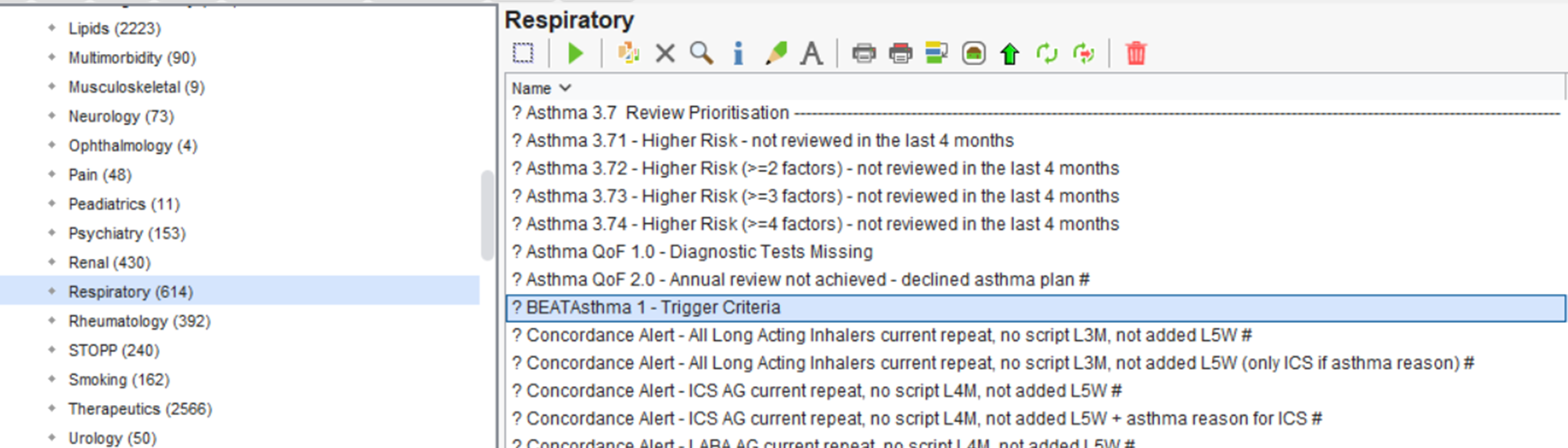
You will first need to run this report. Right-click on this report and press ‘Run’. This may take a few moments depending on the size of your unit’s population.
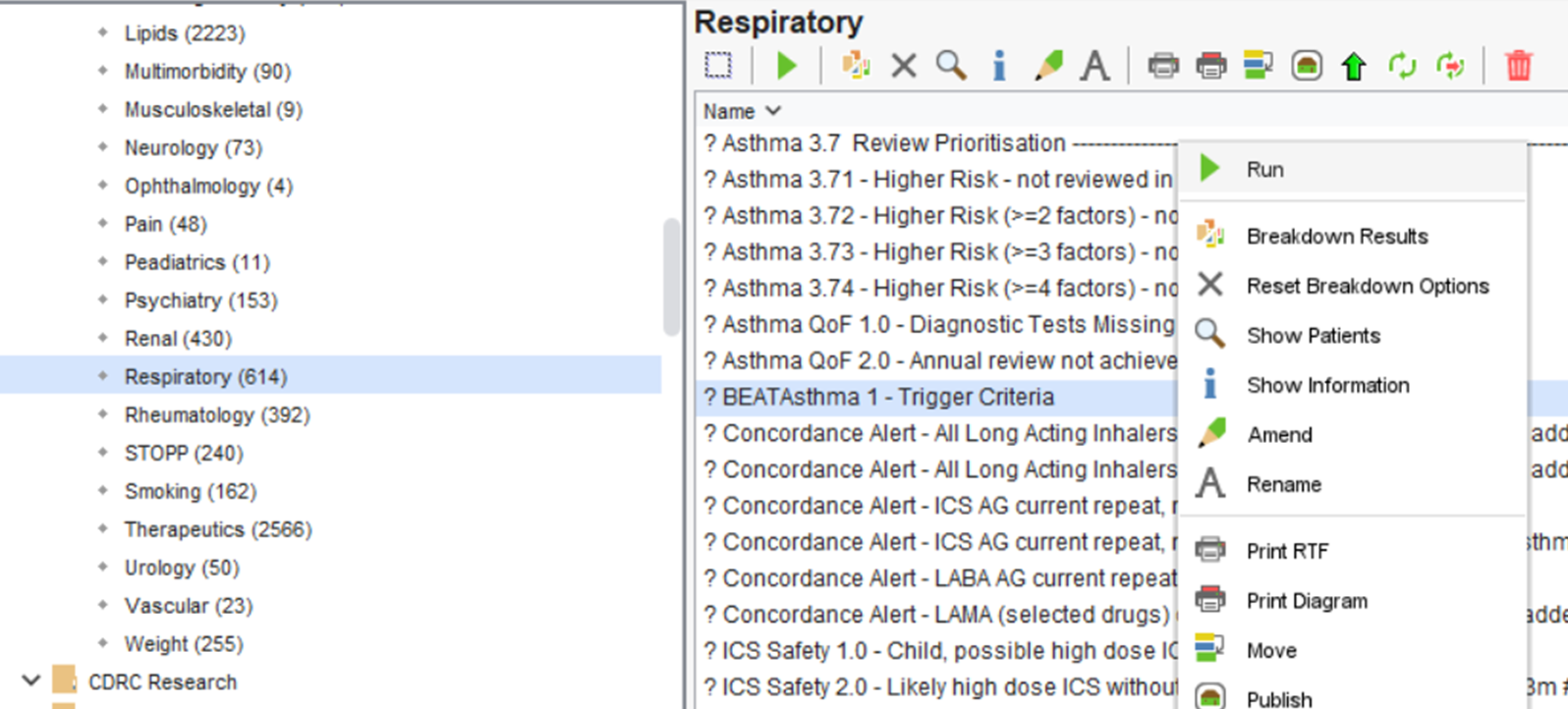
Once this report has been ran, you can view the patient’s returned.
To do this, right-click on the ? BEATAsthma 1 – Trigger Criteria report and click on ‘ Show Patients’.
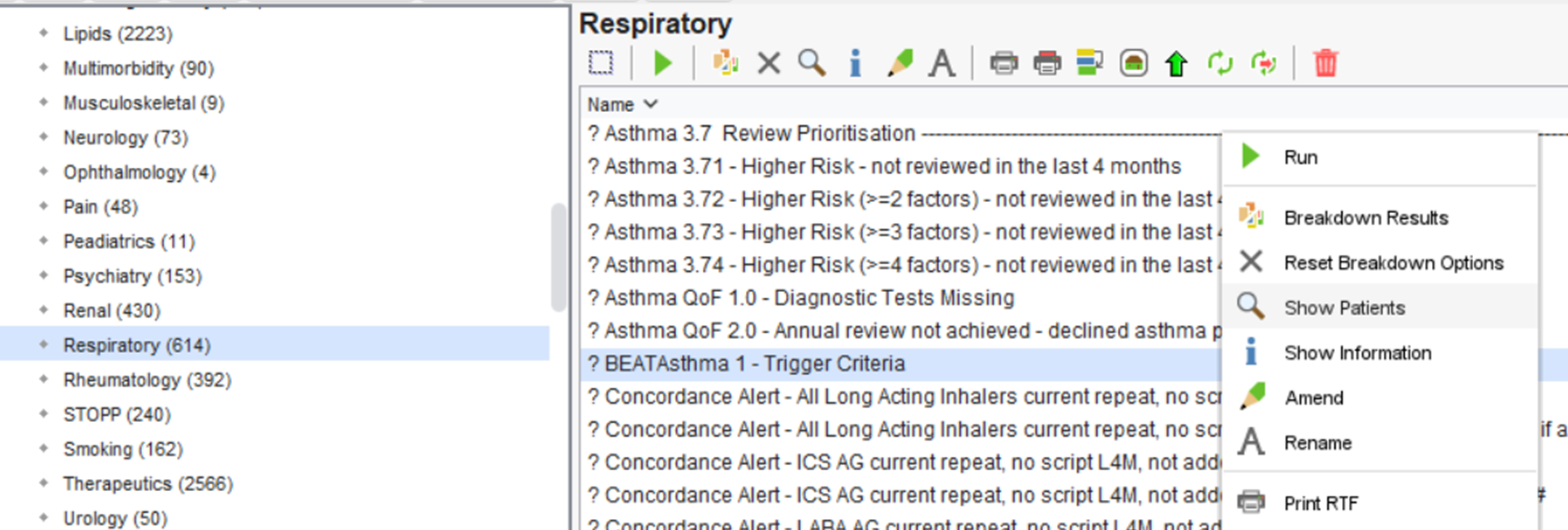
This will show the patient’s identified from this search.
You can view, and save this patient list as an Excel document by clicking on ‘Save All Pages to CSV’.

This will automatically open up an Excel spreadsheet with the patients identified from this search. If you send this patient list, please ensure all patient identifiable information is removed prior to sending.
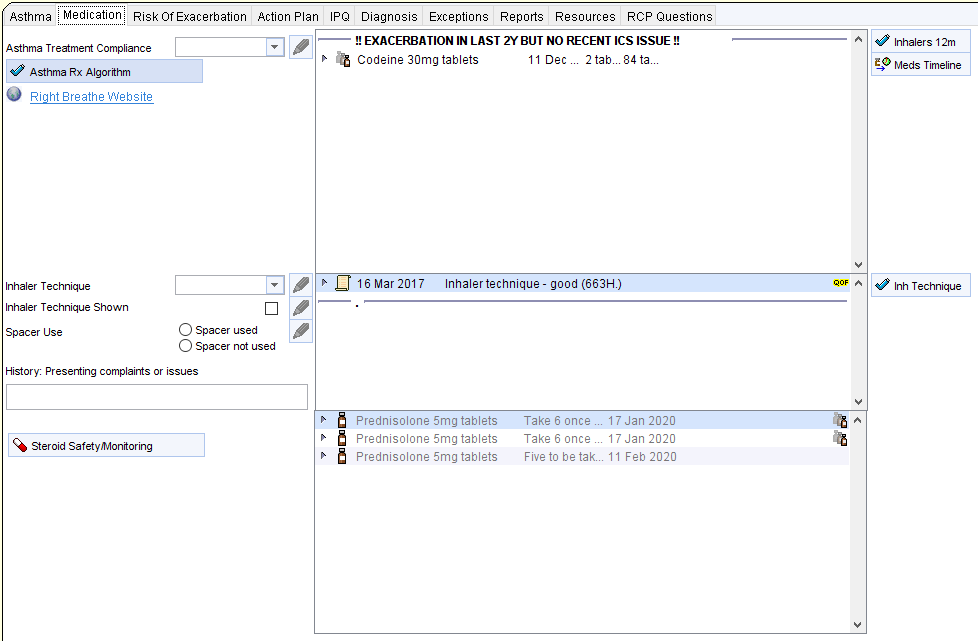
Beat Asthma+ Template
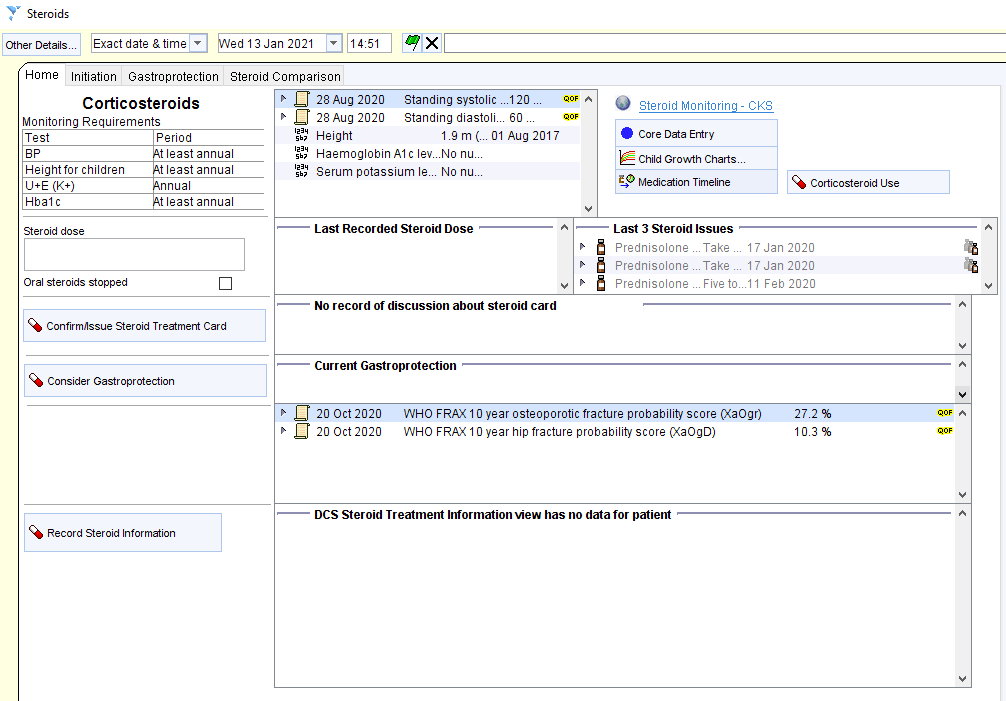
In addition to the Beat Asthma+ Report above, the CDRC has developed an Asthma BEATAsthma Data Entry Template available to all units that are a member of the DCS organisational group on SystmOne.
To access this template, with an identified patient’s record retrieved, using the search bar in the lower left-hand corner of the SystmOne screen, type in Asthma BEATAsthma and select the highlighted template.
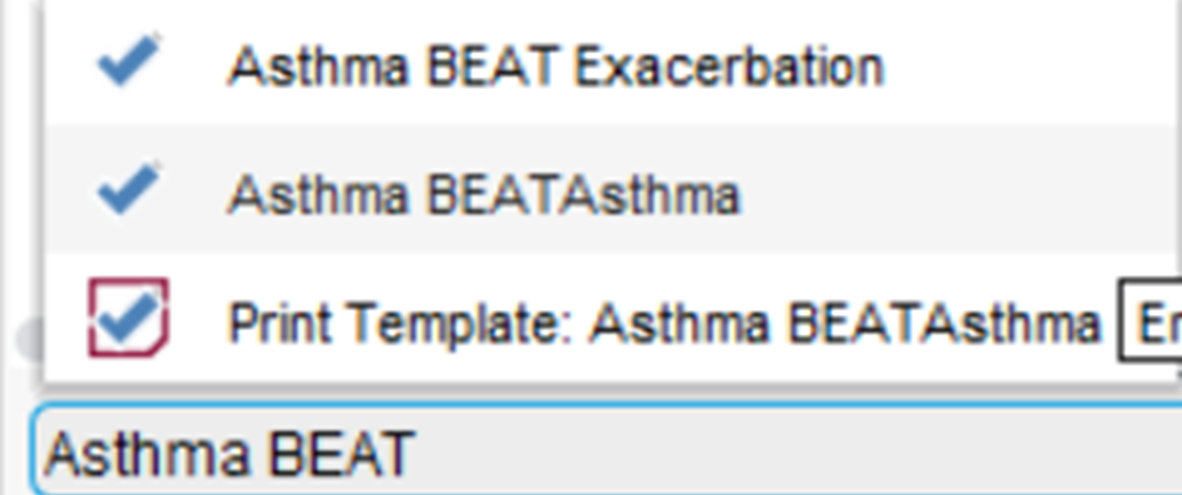
This template has a number of pages which provide valuable information and allows you to input information directly onto the patient’s record.
Further CDRC Asthma Reports
CDRC has additional reports which are not specific to this project but are specific to patients with asthma and can be used to identify higher risk patients, these reports are found in the folder CDRC Quality > Respiratory.
- ? Asthma 3.1a >12 SABA in last 12m
- ? Asthma 3.1b >6 SABA in last 12m
- ? Asthma 3.1c 7-11 SABA in last 12m
- ? Asthma 3.2 LABA on repeat and no ICS #
- ? Asthma 3.3a Likely Severe Asthma (high dose ICS, or LAMA or aminophylline or biologics
- ? Asthma 3.3b Likely Moderately Severe Asthma #
- ? Asthma 3.4 – >1 Exacerbation in last 12m.
- ? Asthma 3.5 Admission or exac in past 2 years but no ICS issued in last 3m #
- ? Asthma 3.6 Latest ACT <20 #
Further information on these reports can be found here.
Get in touch
If you have any questions regarding access, or the use of the CDRC resources, please get in touch: contact-CDRC@ahsn-nenc.org.uk
SUBSCRIBE
Keep up to date with CDRC Precision events and new resources

![Asthma Exacerbation Questionn
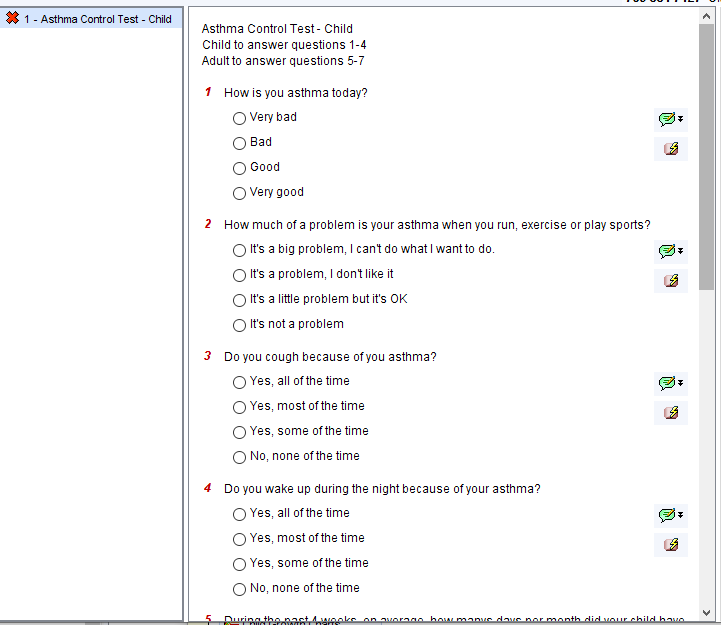
Asthma Exacerbation Questionnaire
11
In the past 4 weeks, how many times a day on average did you use a reliever?
How long does a reliever last (in weeks)?
How many asthma attacks in the past 12 months?
How many courses of prednisolone in the past 12 months?
How many urgent care or A+E attendances for asthma in the past 12 months?
How many hospital admissions for asthma in the past 12 months?
In the previous 12 months have you been admitted to HDL] or ITO?
O Yes
O No
In the past 12 months have you needed M drugs for your asthma?
O Yes
O No
Any other comments
Finish](https://cdrc.nhs.uk/wp-content/uploads/2021/01/image-6.png)