Safe Prescribing of Denosumab
The CDRC module for Denosumab prescribing aims to maximise safety and concordance with guidelines for using this drug.
Set-up
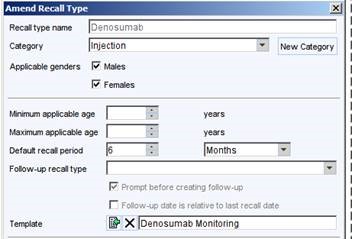
- Create a Recall Type with the following properties
- Ensure that all patients who are receiving denosumab have this medication included on their repeat medication list. This forms your denosumab ‘register’. The quality search CDRC Quality > Rheumatology > DENOS02 will identify patients who have been given an acute script for denosumab in the previous 6 months but do not have this on their repeat medication. DENOS03 will identify those who had a script between 6 and 10 months ago but not currently on their repeat medication list: this might be because they have stopped the denosumab or been lost to follow up.
- Ensure all patients on repeat denosumab have a denosumab recall. The DENOS04 will identify patients who need to have a recall added (NB will not function fully until the day after you have completed step 2.
Recall
A specific report will identify patients who are due to have a denosumab injection within the next two weeks (or have not yet attended for an overdue injection). This report can be found at:
CDRC > Recall Setup > Recall Injection Denosumab (before two weeks hence)
Alternatively, you can use a report that identifies patients who need any regular injection e.g. GnRH analogues, depo-provera, B12. This report can be found at:
CDRC > Recall Setup > Recall Any Injection
You could also create your own bespoke report to include any combination of the injection recalls available. It is a good idea to create a batch report so these reports are run weekly and lead to an automatic task to an administrator to ensure that an appointment(s) is/has been made for injection and/or monitoring and that any appropriate medications are in stock.
Alerts
Patients with denosumab on repeat prescription will have a ‘drugs requiring monitoring’ icon:

Patients on denosumab with up to date monitoring:

Patients on denosumab but monitoring is out of date
Clicking on the icon will launch the DRM (drugs requiring monitoring) template showing which relevant drugs the patient is on.
Patient Review
From the nursing master template click on the Denosumab monitoring button.
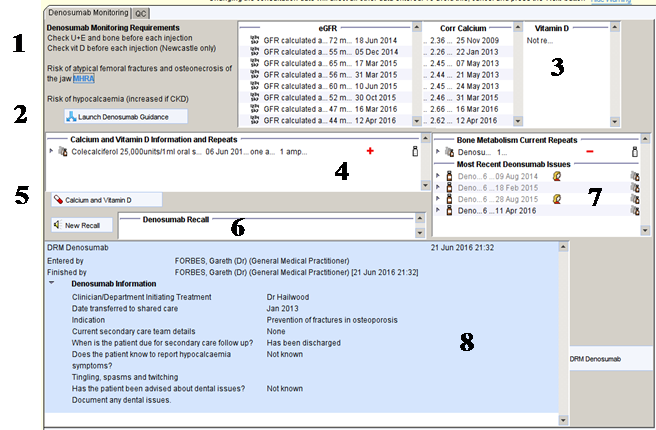
- Description of monitoring requirements
- Link to the local denosumab guidelines
- Most recent monitoring results
- Information about current calcium and vitamin D intake
- Link to vitamin D and calcium template
- Current pending denosumab recall – missing in the example shown
- Current repeat templates for denosumab and previous issues
- Summary of important information
To conduct a denosumab review:
- Ensure that monitoring is up to date
- Check that the information about calcium and vitamin D intake is appropriate
- Check that the information in the bottom panel is up to date and complete. If this panel is empty, click on the DRM Denosumab button to complete the denosumab questionnaire. If this panel already has information but needs updating, right click on the panel and choose Copy Questionnaire then choose Copy Comments then update the information before completing the questionnaire choosing the Save Final Version option
- Administer the injection
- Right click on the Denosumab recall and choose Seen before setting the date for the next recall.
Calcium and Vitamin D
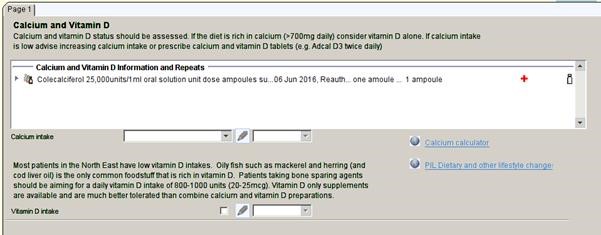
Patients on denosumub must have adequate vitamin D and calcium intake to avoid hypocalcaemia. Calcium and vitamin D intake will be displayed on the denosumab template with a link to the calcium and vitamin D template (above). Here you can calculate calcium intake using an online calculator and record this information. Information about dietary and supplementary calcium and vitamin D intake will then be displayed on the denosumab template.
Quality Control Reports
The following quality control reports can be found in the CDRC Quality > Rheumatology folder or on the QC page of the denosumab template.
| Report Name | Patients Returned |
| DENOS01 Denosumab Register | This counts patients with a repeat script for denosumab |
| DENO02 Denosumab not on repeat | Patients who should have denosumab added to their repeats. Patients who have been given an acute script for denosumab in the last 6 months but this is not included in their repeat medication |
| DENO03 Missed denosumab | Patients who may have been lost to follow up. Identifies patients who have had densoumab between 6 and 10 months ago but it is not included on their repeat list. Patients whose denosumab has been stopped deliberately will appear on this list for four months. |
| DENO04 Denosumab repeat but no recall | Patients on the denosumab register who do not have an appropriate denosumab recall |
| DENO05 Denosumab repeat but no apparent indication | Patients on denosumab without osteoporosis or androgen deprivation therapy – to identify patients where denosumab therapy might be inappropriate |
| DENO06 Denosumab repeat but monitoring up to date | Patients on denosumab without a measurement of calcium and renal function in the last 6 months (and vitamin D for Newcastle patients). You will not need to run this report if you already run the DRM Monitoring overdue report as it will automatically be included in that. |
| DENO07 Denosumab and last calcium low | Patients on denosumab whose last calcium was low |
| DENO08 Denosumab but not adequate calcium/vit D intake | DENOS08 – patient on denosumab who do not have: Calcium supplement or code for high dietary calcium intake within 12m Vitamin D supplement or code for vitamin D intake within 12m |
Ideally these reports should be added to a batch report that sends a regular (monthly or bimonthly) task to an administrator to arrange review of the appropriate patients records.
