The following CDRC resources are available to support safe Lithium Prescribing.
Set-up
The CDRC Lithium monitoring system automatically detects patients with lithium on the repeat medication list who are being issued with lithium. If your unit wishes to leave Lithium off repeat prescription lists (not advised) you can use a specific Lithium recall to flag patients requiring lithium monitoring. You will need to add the recall type: Setup > Data Entry > Recall type
![Amend Recall Type
Recall type name
Shon name
Category
Lithium Shared Care
Applicable genders
Minimum applicable age
Maximum applicable age
Defaut recall period
Recall deadline
Follow-up recall type
Females
years
years
No Deadline
Z] Prompt before creating follow-up
Follow-up date is relative to last recall date
Template
Availabilty
C] Cytology smear dialog (for wating for resuts)
C] Cytology resut dialogs (for scheduling next smear)
Vaccination dialog
C] Contraception dialog
Linked Read codes
Code
-
Description
No codes selected
canc4](https://cdrc.nhs.uk/wp-content/uploads/2022/09/image-282.png)
For each relevant patient, add the Lithium Shared Care recall, ideally with a date very far into the future so the recall doesn’t clog the home screen. This recall is not set to the recall date, it is just used to allow the system to search for patients on shared care. It is not needed if you are issuing repeat lithium scripts.
Reports
The following reports, in the CDRC Quality > Drugs Requiring Monitoring folder, identify patients taking lithium who require assessment
The following reports identify patients taking lithium who require assessment
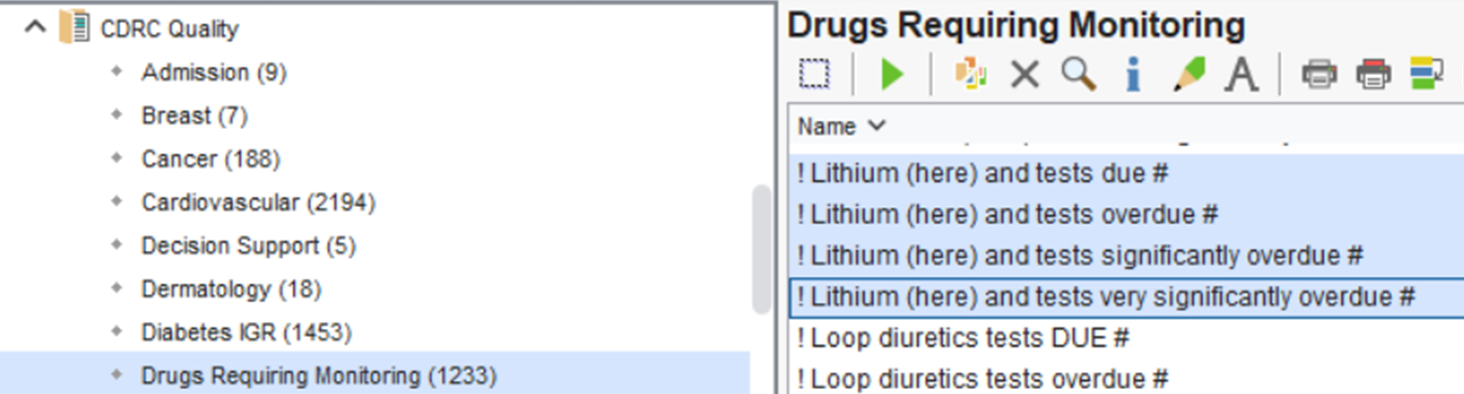
Patients will be included in the CDRC Lithium monitoring system (and these reports) if:
- They have lithium on their repeat prescription and it has been issued in the last three months
OR
- A current pending recall exists with the exact name Lithium Shared Care
Patients appear in these results at the following points
| Usual Test Frequency | ||
| Three monthly | Six monthly | |
| Test due | 77 days | 5 months |
| Test overdue | 84 days | 6 months |
| Test significantly overdue | 89 days | 7 months |
The results from these reports can be used as a stand-alone system for Lithium or as part of the integrated CDRC drugs requiring monitoring system.
Testing frequency for renal function automatically takes into account the most recent renal function/CKD diagnoses.
Patient Status Alerts
The following icons are shown for patients who are in the CDRC Lithium monitoring system depending on whether the testing is up to date or not. Clicking on the icon will open the DRM template where there is a link to the lithium template.


Lithium Template
How to Access:
In the lower left hand corner use the search bar, type in ‘Lithium – CDRC’ and select the template:
Alternatively, press F12 and search for ‘Lithium – CDRC’, this will open the aforementioned template.
Lithium Template
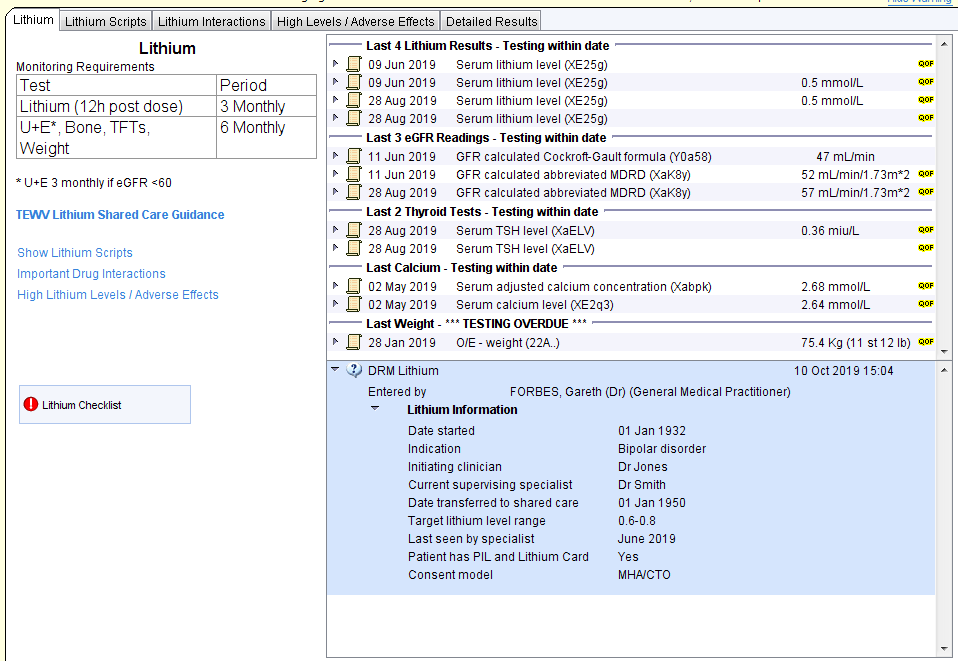
The Lithium template shows key information about monitoring requirements and recent tests.
For each test one of three statuses will be displayed:
- Testing is within date
- Testing is overdue
- Monitoring is not taking place at this organisation
There is a link to the shared care guidelines.
Important drug interactions will be displayed with particular emphasis on RAS drugs, thiazides and NSAIDs. There is a link to more detailed information about drug interactions.
There is a section to record a checklist of important information regarding Lithium.
Lithium Checklist
The Lithium checklist is a space where important questions about Lithium prescribing, responsibility and safety can be recorded. To update the checklist (e.g. if the target range changes), right click on the panel showing the most recent answer and choose Copy Questionnaire then Copy Comments. The old answers will be copied forward and can be updated.
Click Save Final Version once complete.
![Save for Future Editing
Lthium In for mation
Save Final Version
use Previous Answers
Lithium Information
Date started
01 Jan lg32
Indication
Bipolar disorder
C] Depression
C] Other
Initiating clinician
Dr Jones
Cancel
5 Current supervising specialist
Dr Smith
Date transferred to shared care
01 dan lgso
Target lithium level range
0 6-08
Last seen by specialist
June 201g
A safety booklet for patients can be found here but it is better to give out a preprinted pack
Patient has PIL and Lithium Card
Yes
O
Consent model
Informed consent
O
O MHAJCTO](https://cdrc.nhs.uk/wp-content/uploads/2022/09/image-286.png)
Lithium Pop-up Alert
As of October 2019, this popup is only enabled for practices in North Durham. Please contact CDRC to add your area.
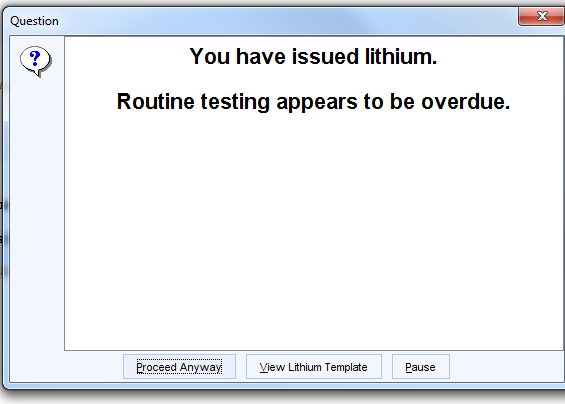
If any Lithium script is issued when testing is overdue, the following alert will be displayed. There is a link to the template to show what is missing.
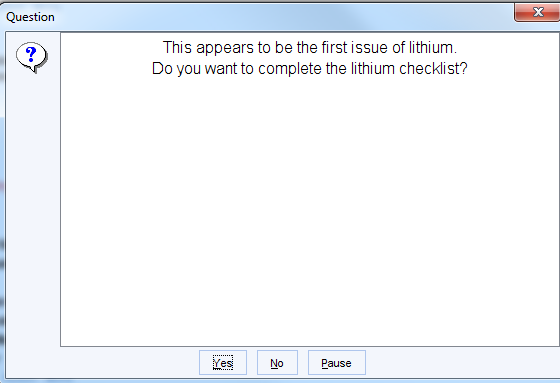
This alert will not trigger, the first time the Lithium is issued, when instead the following message will be displayed.
NB. This alert does not trigger for test patients or patients who are not fully registered patients, such as temporary residents.
