COVID-19 Vaccination
The CDRC Covid19 vaccination system is designed to help maximise uptake of Covid19 vaccination whilst minimising the burden on general practice.
Spring 2024 Booster
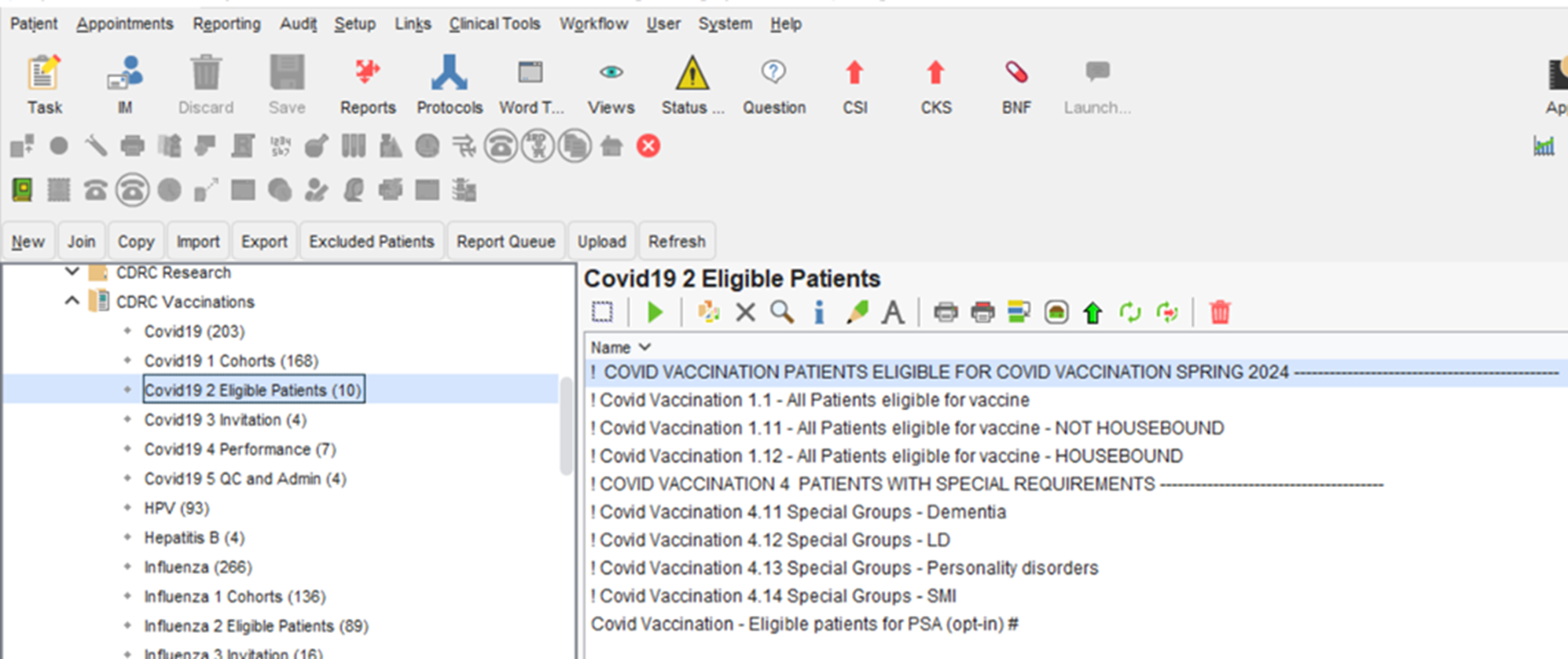
Patients eligible for their Spring 2024 COVID19 Booster can be found in the folder CDRC Vaccinations Covid19 2 Eligible Patients.
These reports will identify patients who are eligible with specific reports for Special Groups.
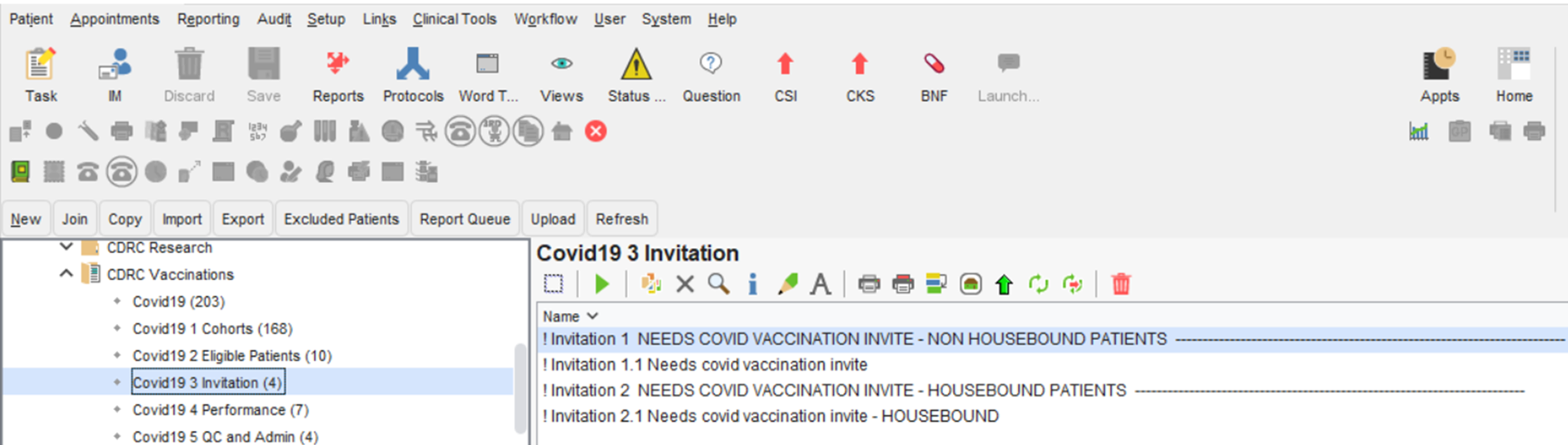
Patients who have not had three invitations for their booster this season, and require an invite can be found in the folder CDRC Vaccinations > Covid19 3 Invitation
COVID19 Patient Identification
The CDRC Covid19 System identifies patients who might be eligible for a covid19 vaccination based on the Enhanced Service specification, Green Book and JCVI guidance.
CDRC will identify patients who may be eligible for Covid19 vaccination but it is up to the individual clinician to confirm that they should receive it.
Identifying Patients Who Are Likely To Be Eligible
- Patient Flag
Patients who might be eligible for their first vaccination will have this patient status alert (green virus particle) if your unit has opted in to them:

Eligible patients will be flagged unless:
- They have declined the vaccine in the last 12 months
- They are on the palliative care register with GSF C/D status
- They have an ‘expiring exception’ in the last 12 months e.g. vaccination not indicated
- They have had covid19 infection in the last 4 weeks (or 12w for healthy 12-15y)
- Shingles vaccine in the last 7 days
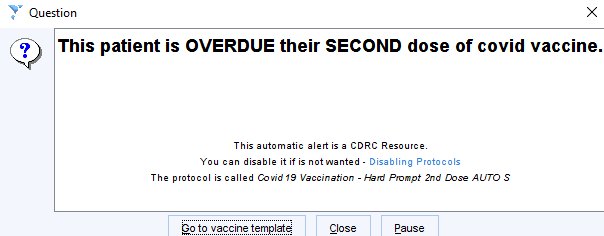
- Pop-up Alert
Eligible patients will display the following pop-up if your practice has opted in to this system
- Patient Searches
![coac Archive (18)
coac contracting (249)
coac Groups (1467)
coac Medicines (13)
coac Performance (298)
coac Population Heath (26)
coac aualty (9857)
coac Research (1 Sl 2)
i] coac Vaccinations (1730)
• covidlg (415)
• Covidlgl cohorts (142)
ovidlg 2 Eligible Patients (87)
• Covidlg 3 Invtation (110)
• Covidlg 4 Performance (128)
• Covidlg S Quality Control (4)
HPV (21)
Hepattis a (4)
• Influenza (400)
• MMR
• Mengingtis (30)
Pneumococcal (161)
• Shingles (129)
• copl (2)
• COVID (2)
• COVID vaccine (2)
COVID1g At Risk (503)
COVID1g At Risk Patients vs (330)
COVID1g At Risk Patients v4 (258)
COVID1g Vaccination (7)
Name v
CovidVaccination 1 Appears eligible for 1st dose - all patients 12y+
Covid Vaccination 1 Appears eligible for 1 st dose - all patients 1 2y+ (except healthy 12-1 5y)
Covid Vaccination 2 Appears eligible for 2nd dose
Covid Vaccination 2M Appears eligible for 2nd dose -AZ
Covid Vaccination 21 Appears eligible for 2nd dose - Moderna
Covid Vaccination 2Z Appears eligible for 2nd dose - Pfizer
Covid Vaccination 3 Appears eligible for 3rd primary dose
Covid Vaccination 4 Appears eligible for booster (Cohorts 1-12)
Covid Vaccination 4M Appears eligible for booster (3m after 3rd dose)
Covid Vaccination 5M All Patients eligible for vaccine
Covid Vaccination 5M 1 All Patients eligible for vaccine -AZ
Covid Vaccination All Patients eligible for vaccine - Moderna (full dose)
Covid Vaccination 5M 22 All Patients eligible for vaccine - Moderna (half dose)
Covid Vaccination 5M 3 All Patients eligible for vaccine - Pfizer
Covid Vaccination 51 All Patients eligible for vaccine (except healthy 12-15)
Covid Vaccination - Eligibility - Hard Prompt- 1st dose (except healthy 12-15) #
Covid Vaccination - Eligibility- Hard Prompt- 2nd dose due (opt-in) #
Covid Vaccination - Eligibility- Hard Prompt- 3rd dose (opt-in) #
Covid Vaccination - Eligibility- Hard Prompt- Booster*
Covid Vaccination - Eligible patients for PSA (opt-in) #
-65) #
Covid Vaccination - Identification
Covid Vaccination - Identification
Covid Vaccination - Identification
Covid Vaccination - Identification
Covid Vaccination - Identification Cohort 01
- Care Home Resident
Covid Vaccination - Identification Cohort 04
Covid Vaccination - Identification Cohort 04M - Cumulative
Cohort 02 -
02
Cohort 03- 75-7g
03
Covid Vaccination - Identification Cohort 04M - Cumulative - 70-74 or SPL with appt*
- Cumulative
- Cumulative
- 70-74 or SPL
- 75-7g#
- 70-74 SPL#](https://cdrc.nhs.uk/wp-content/uploads/2023/02/image-9.png)
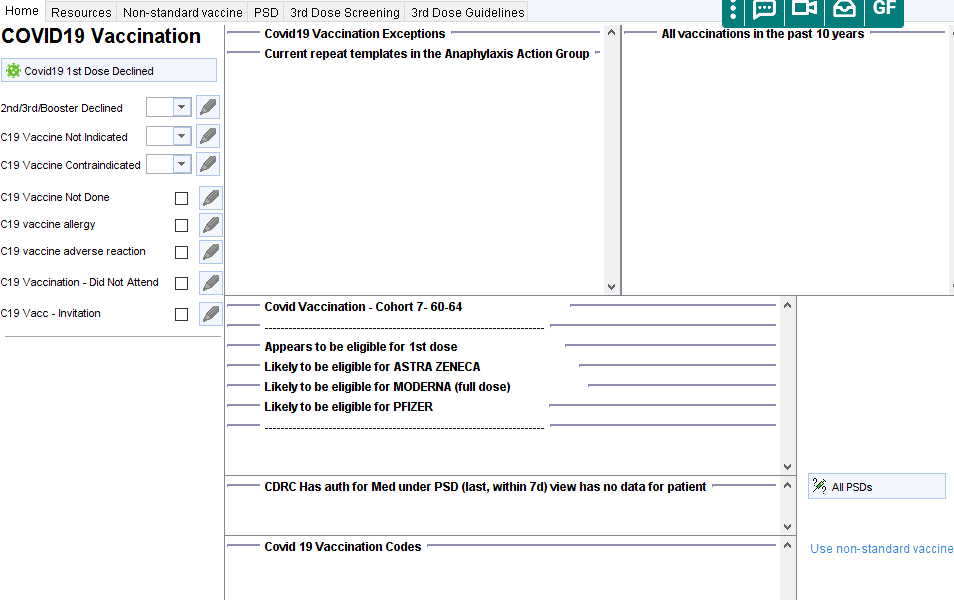
Identifying Why A Patient Is Eligible
Click on the covid19 icon to open the Covid19 vaccination template. The central panel will display the vaccination cohort the patient is in, which dose appears to be due, which brands of vaccine are likely to be suitable, any relevant long term conditions and other appropriate warnings such as recent shingles vaccination or covid infection.
To identify the specific Read code(s) that have triggered eligibility for influenza vaccination, right click on the syringe icon and select Why is this Alert on this Patient? You can then expand the report

Patient Invitation
Patients can be invited for their covid vaccination in a number of ways.
Covid19 Vaccination
- Opportunistic invitation – eligible patients will be identified by a C19 icon (if practice opted-in) below the demographics box, on the home page or on the LTC Master Template. Clicking on the icon will launch the covid vaccination template. This alert will disappear after vaccination, refusal or addition of other exception code.


- Using the invitation searches in the CDRC Vaccination>Covid19 3 Invitation report folder
The reports are separated into housebound and non-housebound patients
Patients are excluded from these reports if:
- Vaccination has been declined in the last 12 months
- Vaccination not indicated or contraindicated codes are recorded in the last 12 months
- Patient has had an invitation in the last 2 weeks
- GSF C/D Palliative care stages
- Covid19 infection in the last 4 weeks (or last 12 weeks for healthy 5-15 year olds)
- Shingles vaccine in the last 7 days
NB – patients who should not have a second dose of Astra-Zeneca vaccine (PHx of venous sinus thrombosis, thrombophilia, heparin-induced thrombocytopenia) will be excluded from the AZ report
| Search | Patients |
| 1.1 | Non-housebound patients aged 12 or over OR 5-11 with an at risk condition who have yet to have any vaccine |
| 1.11 | Non-housebound patients in 1.1 who are higher risk (over 50 or high risk condition) |
| 1.2 | Non-housebound healthy 5-11 year olds who have yet to have any vaccine |
| 1.3 | Eligible housebound patients who are yet to have any vaccine |
| 2.1 | Non-housebound patients who are due a 2nd dose |
| 2.11-2.13 | Non-housebound patients who are due a 2nd dose, divided by initial vaccine type |
| 2.2 | Housebound patients who are due a 2nd dose |
| 2.4 | Searches to identify all patient you are due or will be due second dose – see information about planning searches later in this section |
| 3.1 | Non-housebound patients who are due a third primary dose as they were immmunosuppressed when they had their first or second dose (only functions if the CDRC method of flagging these patients was used) |
| 3.2 | Housebound patients who are due a third primary dose as they were immmunosuppressed when they had their first or second dose (only functions if the CDRC method of flagging these patients was used) |
| 4.1 | All Non-housebound patients due for a booster – this will include many patients who have not responded to multiple invitations for boosters in the past |
| 4.11 | Non-housebound patients due for their 1st booster – most of these patients will have not responded to multiple invitations for boosters in the past |
| 4.111 | Patients in 4.11 who are higher risk (more important to target) |
| 4.12 | Non-housebound patient due for their 1st booster after three course primary vaccination for immunosuppressed patients (only functions if the CDRC method of flagging these patients was used) |
| 4.13 | Non-housebound patients who are due for an Autumn 2022 booster – this does not include patients who have never had a booster – use 4.1 to identify patients who need their first booster OR and Autumn booster |
| 4.21 | Housebound patients who need a booster (1st booster or Autumn booster) |
| 4.22 | Housebound patients who were immunosuppressed at time of 1st or 2nd vaccine who need a booster following their third primary dose (only functions if the CDRC method of flagging these patients was used) |
| 5.1 | All non-housebound patients who are eligible for a vaccine (from 1.9.22) |
| 6.1 | All housebound patients who are eligible for a vaccine (from 1.9.22) |
| 6.2 | All housebound patients who are eligible for or will become eligible for a vaccine (from 1.9.22) – see later information |
More information about eligibility, vaccine dose due, vaccine brand recommended and relevant warnings can be seen on the Covid19 Vaccination template
Invitations can be sent via the S1 Communications Annexe, SMS texting, email, telephone or letter. Add the code Severe acute respiratory syndrome coronavirus 2 vaccination invitation short message service text message sent Y211f to indicate invitation. This will remove the patient from the invite list for 2 weeks.
The SMS invitation code is the only one available – add an alternative method of invitation as free text if applicable. This is not ideal but repeated requests for more codes to NHSD have been ignored.
Running these searches at fortnightly intervals will ensure that you invite all the eligible patients until they have been vaccinated or a reason for not vaccinating has been recorded.
Special Groups
There are searches to identify patients in specific groups, to help more specific targeting:

Capacity Planning
The following searches will allow for more detailed second dose planning e.g. to estimate the number of eligible patients in the future
These searches show all patients who are or will be due a second dose or a booster.
4.31 and 4.32 identify people who will be due the first booster
4.41 and 4.42 identify people who will be due any booster from Autumn 2022 – including a 1st booster or a spring booster.
NB these searches do not exclude the following, so are not suitable for creating lists of people to invite.
- Patient has had an invitation in the last 2 weeks
- GSF C/D Palliative care stages
- Covid19 infection in the last 4 weeks (or last 12 weeks for healthy 12-15 year olds)
- Shingles vaccine in the last 7 days
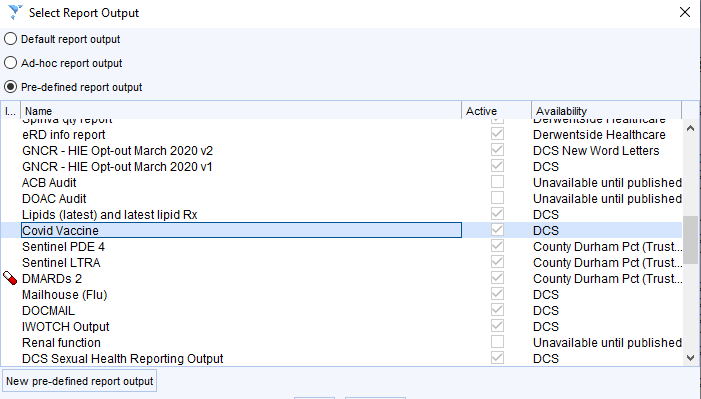
Information for capacity planning can be obtained in two ways:
- Use a Report Output
Show the patients from the relevant search
Choose Select Output then Pre-defined Output then choose Covid Vaccines
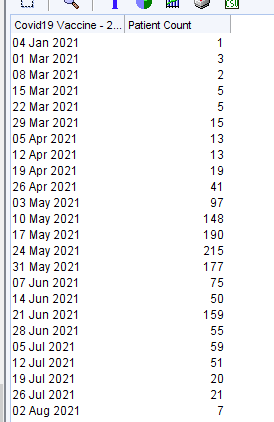
A table of patients will be shown with data for: last vaccine date; if patient has been flagged as needing 3rd primary dose; 1st vaccine date; 2nd vaccine date; any booster vaccine dates. This table can be saved as a csv if needed.
| NHS number | Name | Name | Postcode | (Blank) | (Blank) | (Blank) | (Blank) | (Blank) | (Blank) | (Blank) | Last covid vacc | 3rd primary dose patient | 1st Vacc | 2nd Vacc | 3rd / Booster Vaccs (1) | 3rd / Booster Vaccs (2) | 3rd / Booster Vaccs (3) | 3rd / Booster Vaccs (4) |
| xxxxx | xxxxx | xxxxx | xxxxx | 01-Dec-21 | 17-Feb-21 | 22-Apr-21 | 01-Dec-21 | |||||||||||
| xxxxx | xxxxx | xxxxx | xxxxx | 02-Apr-22 | Covid Vaccine Third Dose | 21-Jan-21 | 09-Apr-21 | 12-Oct-21 | 24-Jan-22 | 02-Apr-22 | ||||||||
| xxxxx | xxxxx | xxxxx | xxxxx | 02-Nov-21 | 05-Feb-21 | 23-Apr-21 | 02-Nov-21 | |||||||||||
| xxxxx | xxxxx | xxxxx | xxxxx | 02-Nov-21 | Covid Vaccine Third Dose | 17-Feb-21 | 28-Apr-21 | 02-Nov-21 | ||||||||||
| xxxxx | xxxxx | xxxxx | xxxxx | 02-Nov-21 | 23-Jan-21 | 12-Apr-21 | 02-Nov-21 | |||||||||||
| xxxxx | xxxxx | xxxxx | xxxxx | 03-Feb-22 | Covid Vaccine Third Dose | 19-Jan-21 | 25-Mar-21 | 28-Oct-21 | 03-Feb-22 | |||||||||
| xxxxx | xxxxx | xxxxx | xxxxx | 04-Nov-21 | 10-Feb-21 | 30-Apr-21 | 04-Nov-21 | |||||||||||
| xxxxx | xxxxx | xxxxx | xxxxx | 04-Nov-21 | 25-Jan-21 | 13-Apr-21 | 04-Nov-21 | |||||||||||
| xxxxx | xxxxx | xxxxx | xxxxx | 05-May-22 | 12-Jan-21 | 30-Mar-21 | 05-Oct-21 | 05-May-22 |
- Use a Search Breakdown
Breaking Down 2nd Dose Planning Searches
For each of the searches you can set the following breakdown options to see all the info you need to plan second doses.
Demographics – name, telephone number etc (if needed)
Covid19 Vaccine – All (code 1/2) # (CDRC Vaccinations / Covid19)
>Event details
>Event week commencing – gives date of first vaccine
>Event done at – gives location of first vaccine(if needed)
Then order by Event date and Event done at.
NB – Patients who are due second AZ but where second AZ is contraindicated are excluded from the AZ report (e.g. history of heparin induce thrombocytopenia)
If you need a breakdown of all patients who will be due a second dose – use the ‘All vaccine types’ report and use the additional breakdown options.
Covid19 Vaccine – Moderna Sept 20 – Aug 21 (code or S1 or med) ONLY #/ (CDRC Vaccinations / Covid19) > Reporting ID – patients who had Moderna as first dose
Covid19 Vaccine – Oxford AZ Sept 20 – Aug 21 (code or S1 or med) ONLY #/ (CDRC Vaccinations / Covid19) > Reporting ID – patients who had Oxford AZ as first dose
Covid19 Vaccine – Pfizer Sept 20 – Aug 21 (code or S1 or med) ONLY #/ (CDRC Vaccinations / Covid19) > Reporting ID – patients who had Pfizer as first dose
Covid19 Vaccine – Type – More than 1 #/ (CDRC Vaccinations / Covid19) > Reporting ID – patients where brand of first dose is unclear (e.g. not recorded or more than one brand recorded)
Covid19 Vaccination – AstraZeneca – patients with clotting contraindications / (CDRCVaccinations / Covid19) > Reporting ID – patients who should not have AZ as second dose
Breaking Down Booster Dose Planning Searches 4.31 and 4.32
For each of the searches you can set the following breakdown options to see all the information you need to plan booster doses.
Demographics – name, telephone number etc (if needed)
Covid19 Vaccine – 2nd dose (code, latest) # (CDRC Vaccinations / Covid19)
>Event details
>Event week commencing – gives date of first vaccine
>Event done at – gives location of first vaccine(if needed)
Then order by Event date and Event done at.
If you wish to highlight the patients with current infection, also tick:
Eligibility – Covid19 Infection confirmed in last 4w # > Strategic Reporting ID > Patient ID
AND
Eligibility – Covid19 Infection confirmed in last 12w – aged 12-17 without risk condition #> Strategic Reporting ID > Patient ID
Breaking Down Spring Booster Dose Planning Searches 4.41 and 4.42
For each of the searches you can set the following breakdown options to see all the information you need to plan the next doses.
The breakdown below shows all people who will become eligible for a covid vaccine follow up dose in Spring to Summer 2022 – 2nd dose, booster, spring booster etc and the date of their last vaccine.
Covid19 Vaccine – (code 1/2/booster) latest # (CDRC Vaccinations / Covid19)
>Coded entries
>Read code description
>Event details
>Event week commencing
Then order by Event date
Patients with Administration of first dose of SARS-CoV-2 vaccine – will need 2nd dose
Patients with Administration of second dose of SARS-CoV-2 vaccine – will need booster dose
Patients with Immunisation course to maintain protection against SARS-CoV-2 – will need 2nd / 3rd (Autumn) booster

Giving the Vaccine
To administer the vaccine click on the C19 icon at the top of the screen. The influenza vaccine
template will open and lead you through the process, adding the correct Read codes.

For patients without the icon, you can launch template from the CDRC Master Template or add it to one
of your own templates, toolbars or clinical tree.
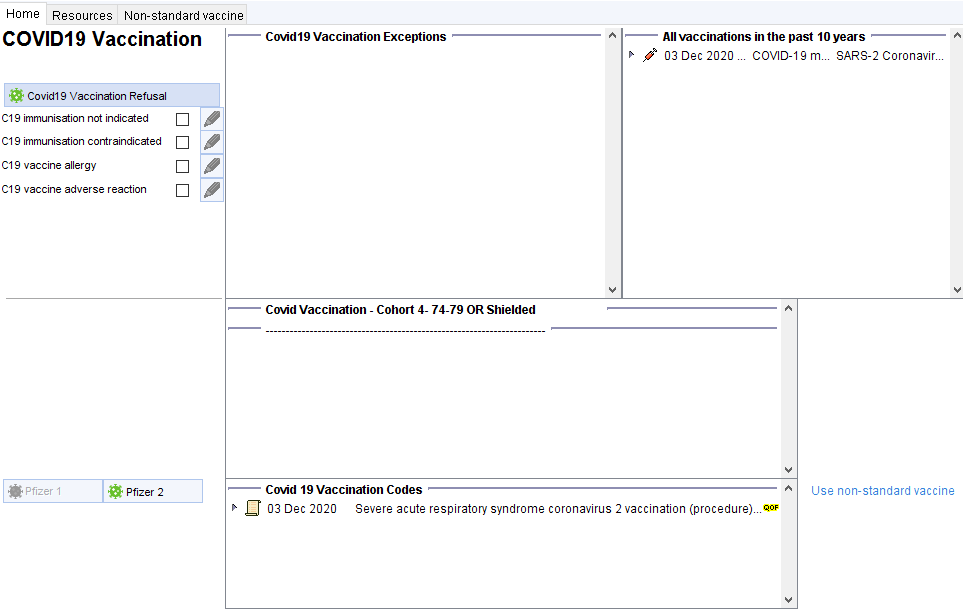
The template features
Section to code exceptions/adverse reactions/refusal. Relevant codes already in the record are shown in the adjacent panel. The far right panel here shows all vaccines administered in recent years
The next sections covers cohorts, reasons for eligibility and warning such as pregnancy.
The final section allows you to record the vaccine. Vaccines that do not appear appropriate will be greyed-out. You can override this by clicking through to the non-standard vaccine tab. This template has been prepared in anticipation that SystmOne will be able to meet MHRA and NHSD standards, possibly in the new year. Until then practices are reminded that Pinnacle is currently the only approved system for the onsite recording of Covid-19 vaccine administration.
The following checks are run when the vaccine protocol is launched
All protocols
- Patient is over 16
- Patient is not pregnant
- Patient does not have history of adverse reaction to covid 19 vaccine
- Patient does not have a record of ‘contraindicated’ or ‘not indicated’ in the past 12 months
- Patient has had any vaccine in the last 7 days
- Patient has had covid19 infection in the last 28 days
- Patient has already completed a course of covid19 vaccination
First vaccination protocols
Patient hasn’t already had a first dose
Second vaccination protocols
The patient has had the first dose of the same vaccine at the appropriate time period e.g. at least 19 days ago for Pfizer.
The PSD questionnaire will then be displayed with automatic warnings.
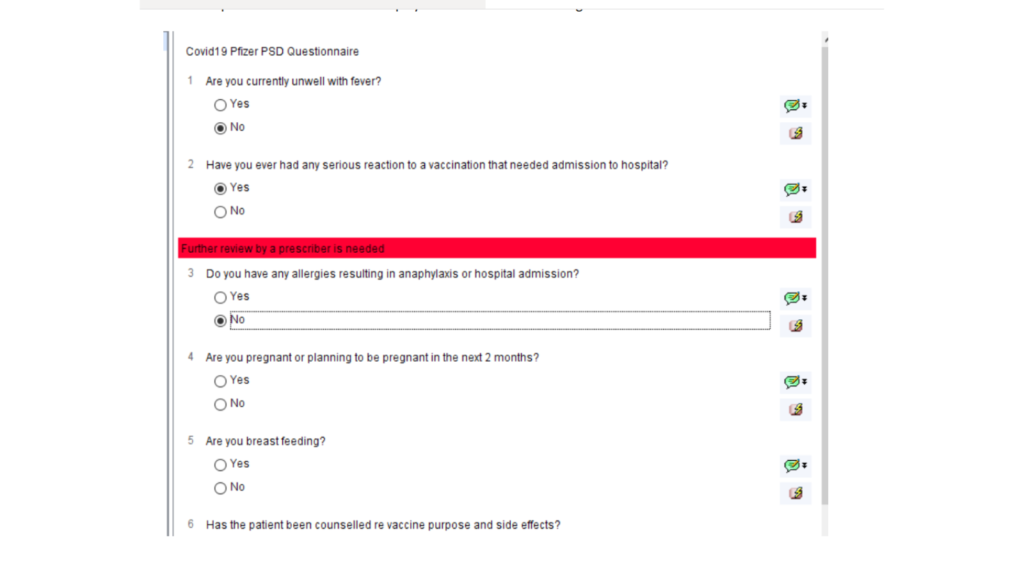
Once the first vaccination is complete, the user will be prompted to book a second appointment for the second dose.
COVID Vaccine Third Dose
Guidance
The guidelines (issued in early September 2021) to identify patients who need a 3rd dose as part of their primary regime are complex (guidance). Some of the information required might not be coded in the primary care record e.g. biologics being issued in secondary care.
The guidance is based on looking for patients with significant immunosuppression at the time of 1st and 2nd doses of vaccine
The following resources can help:
Searches
The following searches will help to identify potentially eligible patients

| 1.1 | Patients who are likely to be eligible for 3rd dose |
| 1.11 | Patients in 1.1 who haven’t been flagged as needing a third dose |
| 1.2 | Patients who might be due for 3rd dose depending on drug doses/timing |
| 1.21 | Patients in 1.2 who haven’t been flagged as needing a third dose |
| 1.3 | Patients who have been flagged by the practice as needing a 3rd dose (see below) |
Patients will only appear in the first four searches once they have had their second dose of vaccine
Screening Potential Eligible Patients
The vast majority of patients in the ‘probables’ 1.1 search will be eligible for a third dose.
Around 1/4 to 1/3rd of patients in the ‘possibles’ 1.2 search are likely to be eligible for a third dose, with another ~10% probably needing some input from supervising consultants regarding hospital treatment such as biologics.
For some patients it will be clear why 3rd dose is needed e.g. transplant or chemotherapy before 1st/2nd doses. For others more information will be needed. There are two tools which may help with this
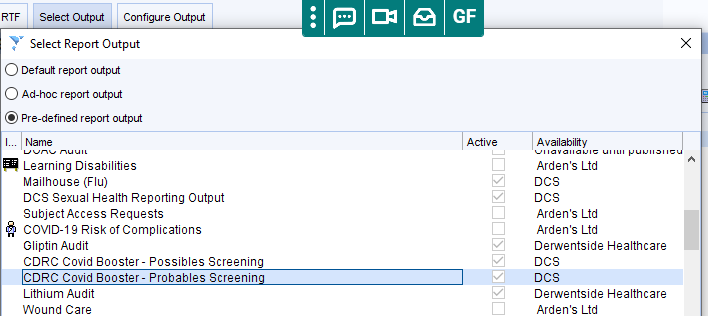
- Report Output – which shows the list of patients with relevant information. From the search, show the patients, then click on the Select Output button, then tick Pre-defined report output and then choose the ‘probables’ or ‘possibles’ report outputs shown below. It will usually be easier to use the export to spreadsheet option to view and manipulate the list.
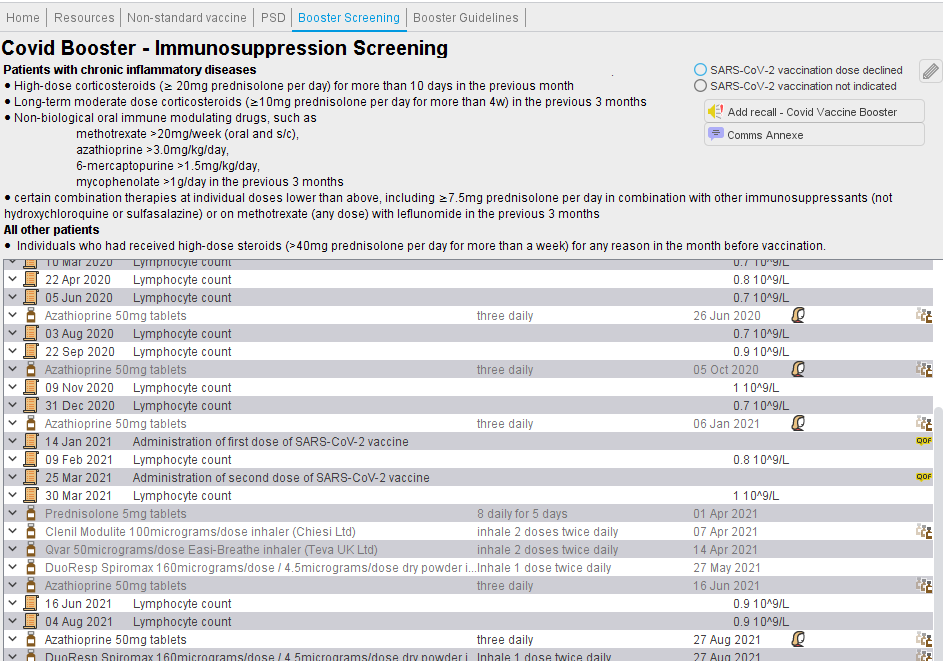
- The Covid19 Vaccination template will help with this screening. The panel will show relevant drug and lymphocyte count information with the covid vaccine administration dates shown. In the example below, the patient was lymphopenic before the first and second doses of vaccine. This template is also useful to view timing and quantity of steroid prescribing in relation to the vaccine doses.
The process can be streamlined by selecting all patients in the screening list, right clicking on the list, then choose Actions > Use Data Entry Template, then choose the Covid19 Vaccination template, and at the next dialogue box add your details in the staff member box. S1 will then open each patient’s record with the template.
Flagging Patients For Third Dose
As there is no Snomed code to indicate need for booster an alternative is to use a recall. Ask an administrator to add a recall type with the name Covid Vaccine Third Dose (spelling and spaces important). Then add a pending recall to any patient who should be offered the booster. This can be done from the screening template or from a patient list in reporting. The date of the recall does not matter. Patients with such a recall will show on the 1.3 search above
NB – a previous version of this guide suggested using the recall name Covid Vaccine Booster. If you have added this recall, it will still be detected by the system. Covid Vaccine Third Dose is a more accurate title for the recall. If you wish to change the recall name you can do so easily Renaming A Recall Type
Inviting Patients
See Patient Invitation above
After Vaccination
Patients who have had a third dose, will need a booster dose sthree months later. To ensure the system is able to recognise these patients, ensure that the Covid Vaccine Third Dose recall is not deleted. You may wish to mark the recall as Seen once the patient has had the third dose so the recall doesn’t flag in the pending recalls screen, but this isn’t essential. In this way you will always be able to show the list of patients who need/needed a third primary dose.
PSD
The following process can help clinicians add a PSD for the covid19 vaccine.
Step 1. – Create a local copy of the PSD report.

As you progress through the vaccination programme you will need to update this report with the current cohort. The initial report is set for cohort 2 stage.
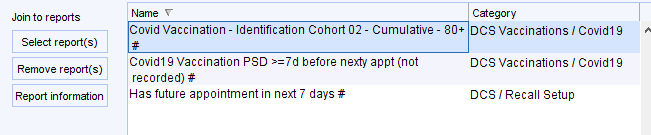
To move this on remove the report highlighted above and replace it with one of these reports
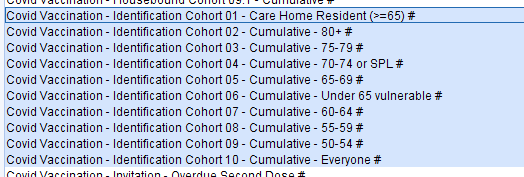
Run this report which will identify eligible patients in the relevant cohorts with an upcoming appointment who do not already have a PSD recorded within 7 days of that appointment. To see which appointments are due, use the Breakdown options to select the appointment details.
![Covidlg Vaccination PSD before nexty appt (not recorded) # (
Has future appointment in next 7 days # (DCS Recall Setup)
Patient Count
Appointments (3)
Appointment arrival time
[3 Appointment booked date
Appointment branch
[3 Appointment cancellation date
Appointment date
[3 Appointment duration (actual)
Appointment duration (booked)
[3 Appointment flags
Z] Appointment location
Appointment status
Appointment time
Appointment wating time (absolute)
Appointment wating time (calculated)
Booked by
Z] Clinician](https://cdrc.nhs.uk/wp-content/uploads/2020/12/image-14.png)
Select and highlight the relevant patients. Deselect any patients who are not suitable for vaccination. Click on the magnifying glass icon to display the selected patients.
At the next screen, click on the scroll to add the PSD
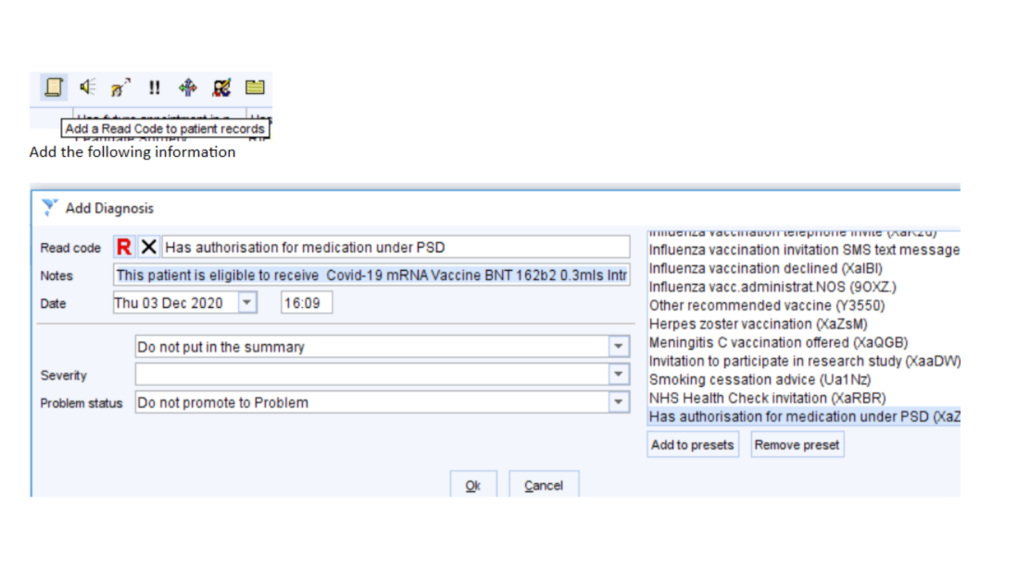
…..using this text
‘This patient is eligible to receive Covid-19 mRNA Vaccine BNT 162b2 0.3mls Intra-Muscular (IM) Injection in accordance with Public Health England Immunisation against infectious disease (Green Guide) and JCVI recommendations for the purpose of protection against COVID-19. Dr Xxxxx Xxxxx ‘
This PSD will then be displayed on the covid19 vaccination template for 7 days
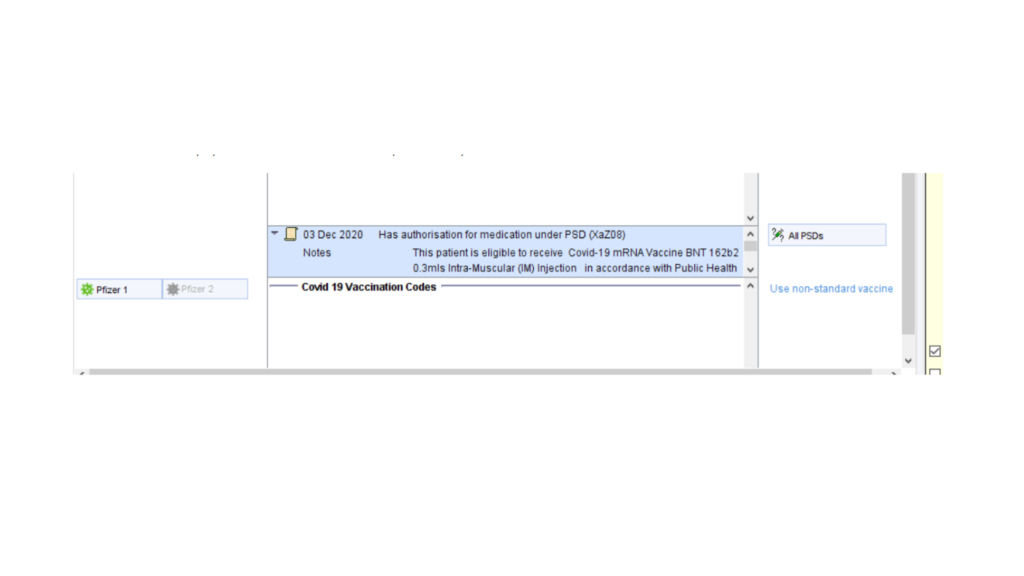
Alternatively, use the PSD tab of the covid 19 vaccination template the add the same information to individual patient records. There is a preset text option for quick entry.
Cohort 6 Issues
TPP/Primis Search Issues
There are significant differences between the searches to identify patient in cohort 6 between CDRC and TPP/Primis
These differences are summarised in the table below.
| CDRC | TPP | |
| Hyposplenism | ||
| Respiratory | Includes any life-threatening asthma episodes Counts any 3 oral steroids prescriptions in the last 3 months | Only counts 3 oral steroid prescriptions if in consecutive months |
| CNS | Must have epilepsy QoF code and be taking epilepsy medication | Includes any codes for epilepsy including some seizure codes which may have been one-off events. Does not look at epilepsy medication. Many patients picked up by this strategy had childhood epilepsy or seizures only. Includes some codes that may have been one-off events e.g. post-concusional syndrome |
| Chronic liver disease | Includes non-alcoholic fatty liver | Does not include NAFLD Does include ‘fatty liver’ which may have been a transient phenomenon Includes resolved hepatitis B and C |
| Chronic Kidney Disease | Includes many glomerulonephritis codes which may have been transient – e.g. minimal change nephropathy Does not take into consideration CKD ‘resolved’ codes | |
| Chronic Heart Disease | Includes many codes for valve disease (often mild or benign) – this does not seem to be indicated according to the JCVI criteria unless 1. congenital, 2. causing significant problems such as heart failure Codes for superficial thrombophlebitis included (likely to be a coding error as these codes are children of venous thrombosis) Retinal vein occlusion is included | |
| BMI >40 | ||
| Serious mental illness | Does not take ‘in remission’ codes into account. | |
| Learning disability | ||
| Diabetes | Does not take ‘in remission’ codes into account | |
| Immunosuppression | Will detect any patients whose hospital provided immunosuppressants/biologics are recorded on their S1 repeat templates | Does not take ‘immunosuppression resolved’ codes into account. |
| Carers** | Only counts ‘non-professional’ carers | Counts professional carer codes as well as non-professional |
The following searches will help practices check any differences between CDRC and TPP searches
The first search identifies people coded as professional carers (‘Carer’) who are not also recorded as informal carers. The recommended code for informal carers is ‘Patient themselves providing care’.
The second search shows all patients identified by the TPP searches but not by CDRC.
Both searches exclude people in cohorts 1-5; those who have been vaccinated already; or where the vaccine has been declined or is not indicated/is contraindicated.
Cohort 6 CDRC Change Log
| 15/2/21 | VTE and AF added Asthma limited to those with history of hospital admission or >=3 courses of steroids in the last year Patients <65 in residential care added |
| 19/2/21 | Lifethreatening asthma attack added Asthma limited to >=3 courses of steroids in the last 3 months |
| 22/2/21 | Epilepsy expanded to included 16 and 17 year olds Neurofibromatosis type 1 and hydrocephalus added to neurological conditions Cardiomyopathy, coarctation of the aorta, LVH and LVSD added to cardiac conditions |
| 24/2/21 | Personality disorder included Eating disorder in the last 5 years included |
| 27/2/21 | Tuberous sclerosis included |
| 4/3/21 | More myeloproliferative conditions added |
Astra-Zeneca Safety
The following searches (CDRC Vaccination > Covid19 1 Cohorts) identify patients who should definitely not have a first or second dose of the Astra-Zeneca vaccine. This is a very small group of patients with a history of venous sinus thrombosis, thrombophilia or heparin induced thrombocytopenia.

The first search identifies all patients in this group.
The second identifies these patients who have yet to have a coronavirus vaccine – so they can be informed not to have AZ and invited for an alternative vaccine.
The third identifies these patients who are due to have a second AZ vaccine. They should be informed not to have a second AZ vaccine and an alternative brand should be administered.
The ‘Consider Invitation for 2nd Oxford AZ vaccine’ searches now automatically exclude patients in the ‘contraindicated’ group described at the top of this page.
Quality Control
There are searches to identify some of the commoner quality control issues.
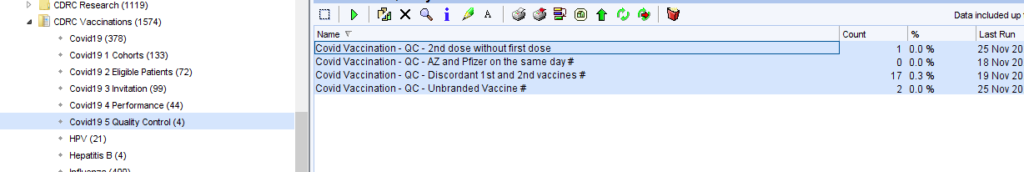
| QC – 2nd dose without first dose | May be due to incorrect coding of the first dose or missing coding of the first dose |
| QC – AZ and Pfizer on the same day | Both vaccines recorded on the same day – highly likely to be an error |
| QC – Discordant 1st and 2nd vaccines | May have been a deliberate decision but may have been due to coding error |
| QC – Unbranded Vaccine | First dose given but brand not specified which makes planning for the second dose more difficult |
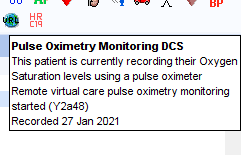
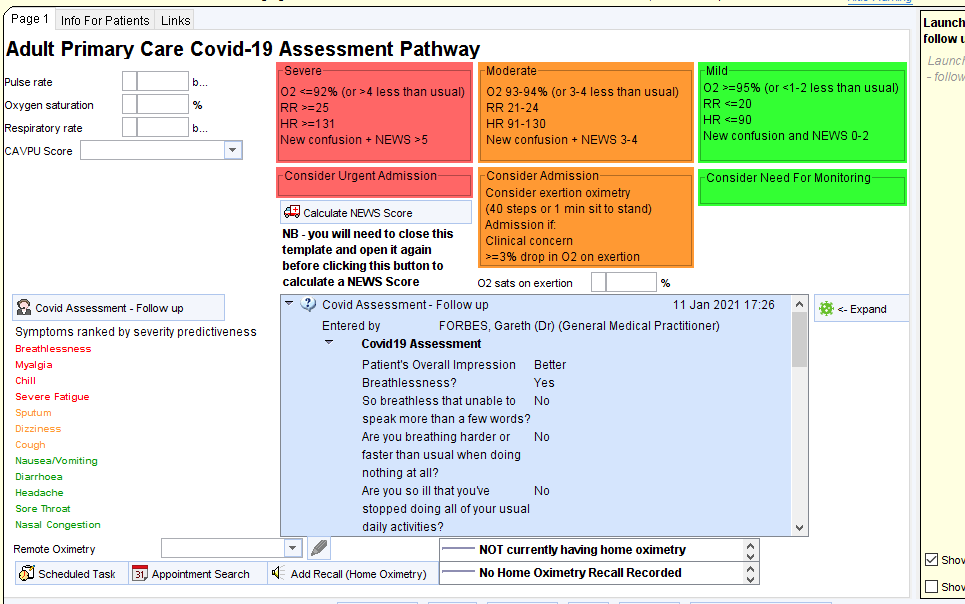
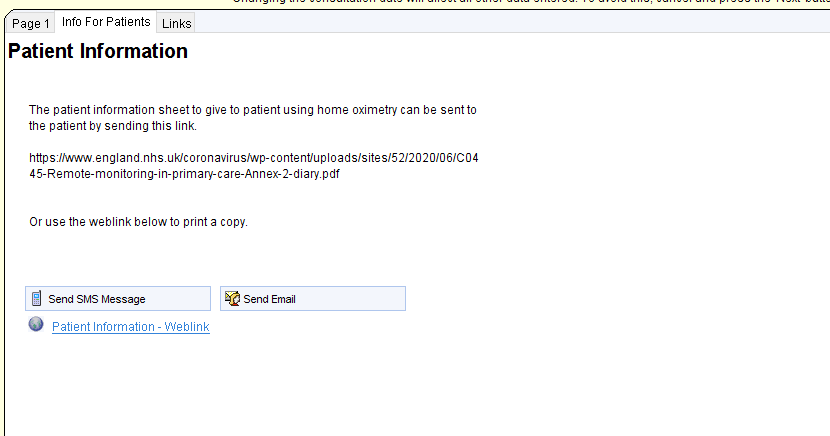
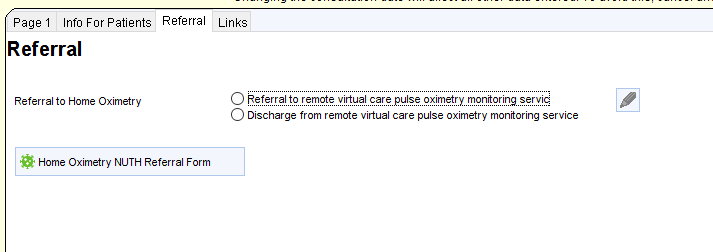
COVID Oximetry@Home
The steps below outline how to use the CDRC Precision COVID19 Oximetry at home guides for SystmOne: